Accelerated aging of skeletal muscle and the immune system in patients with chronic liver disease
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
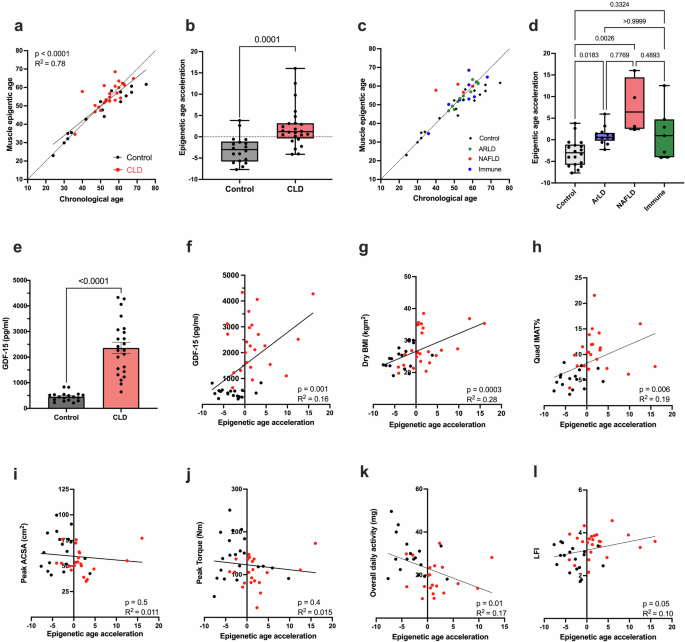
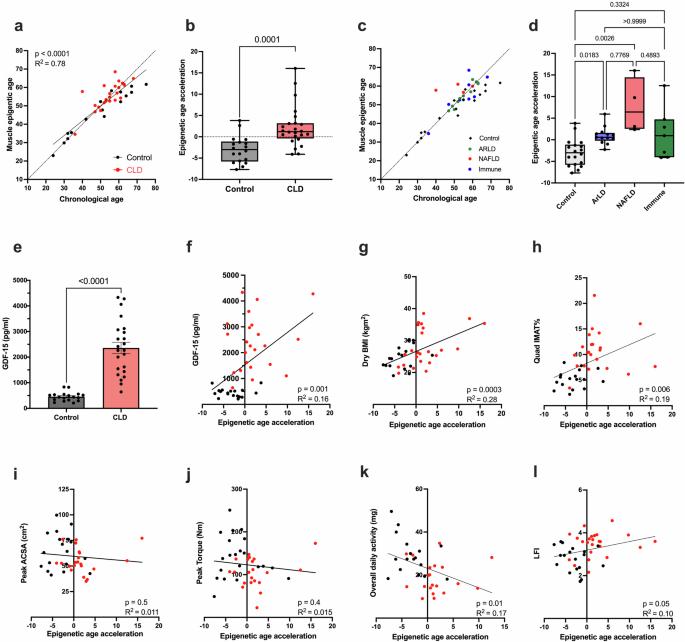
Patients with chronic liver disease (CLD) often present with significant frailty, sarcopenia, and impaired immune function. However, the mechanisms driving the development of these age-related phenotypes are not fully understood. To determine whether accelerated biological aging may play a role in CLD, epigenetic, transcriptomic, and phenotypic assessments were performed on the skeletal muscle tissue and immune cells of CLD patients and age-matched healthy controls. Accelerated biological aging of the skeletal muscle tissue of CLD patients was detected, as evidenced by an increase in epigenetic age compared with chronological age (mean +2.2 ± 4.8 years compared with healthy controls at −3.0 ± 3.2 years, p = 0.0001). Considering disease etiology, age acceleration was significantly greater in both the alcohol-related (ArLD) (p = 0.01) and nonalcoholic fatty liver disease (NAFLD) (p = 0.0026) subgroups than in the healthy control subgroup, with no age acceleration observed in the immune-mediated subgroup or healthy control subgroup (p = 0.3). The skeletal muscle transcriptome was also enriched for genes associated with cellular senescence. Similarly, blood cell epigenetic age was significantly greater than that in control individuals, as calculated using the PhenoAge (p < 0.0001), DunedinPACE (p < 0.0001), or Hannum (p = 0.01) epigenetic clocks, with no difference using the Horvath clock. Analysis of the IMM-Age score indicated a prematurely aged immune phenotype in CLD patients that was 2-fold greater than that observed in age-matched healthy controls (p < 0.0001). These findings suggested that accelerated cellular aging may contribute to a phenotype associated with advanced age in CLD patients. Therefore, therapeutic interventions to reduce biological aging in CLD patients may improve health outcomes. Chronic liver disease, a long-term condition damaging the liver, is causing more deaths worldwide. Patients often develop immune dysfunction and sarcopenia. This study aimed to see if CLD patients show signs of fast biological ageing, particularly in muscles and the immune system. The research compared CLD patients to healthy people, looking at muscle samples, blood samples, and immune cells to check for ageing signs. Results showed that CLD patients have faster biological ageing in muscles and immune cells, with increased epigenetic age and more senescence-associated genes. This suggests that CLD speeds up the ageing process, which could explain the common occurrence of sarcopenia and immune dysfunction in these patients. Future implications include the possibility of developing treatments targeting the ageing process in CLD patients, offering hope for better health and quality of life This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
加速慢性肝病患者骨骼肌和免疫系统的衰老。
慢性肝病(CLD)患者通常表现出明显的虚弱、肌肉疏松和免疫功能受损。然而,这些与年龄有关的表型的形成机制尚未完全明了。为了确定加速生物衰老是否可能在 CLD 中起作用,研究人员对 CLD 患者和年龄匹配的健康对照组的骨骼肌组织和免疫细胞进行了表观遗传学、转录组学和表型评估。结果发现,CLD 患者骨骼肌组织的生物衰老速度加快,表现为表观遗传年龄比实际年龄增加(平均+2.2 ± 4.8岁,而健康对照组为-3.0 ± 3.2岁,P = 0.0001)。考虑到疾病病因,与酒精相关(ArLD)(p = 0.01)和非酒精性脂肪肝(NAFLD)(p = 0.0026)亚组的年龄加速明显大于健康对照亚组,免疫介导亚组或健康对照亚组未观察到年龄加速(p = 0.3)。骨骼肌转录组也富集了与细胞衰老相关的基因。同样,使用 PhenoAge 计算得出的血细胞表观遗传年龄也明显大于对照组(p = 0.3)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


