Profiling protein–protein interactions to predict the efficacy of B-cell-lymphoma-2-homology-3 mimetics for acute myeloid leukaemia
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
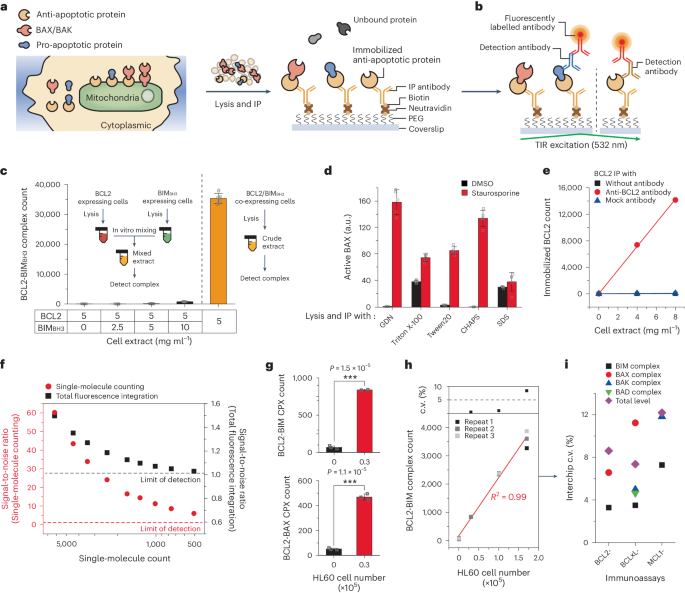
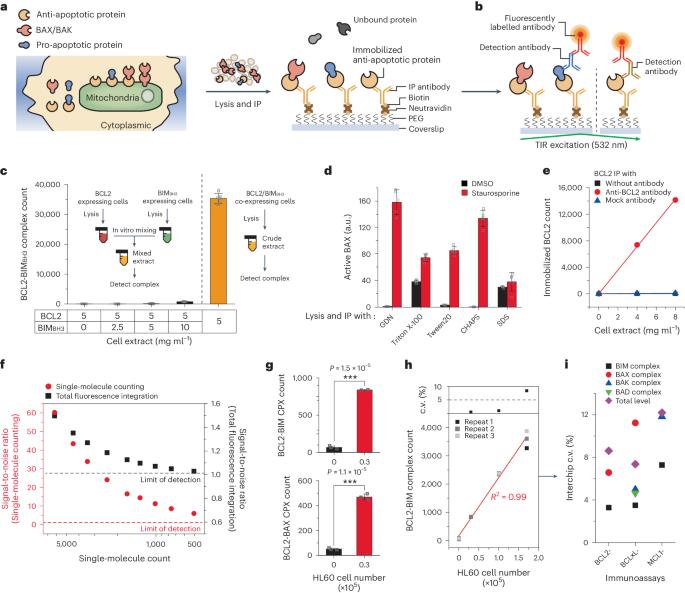
B-cell-lymphoma-2 (BCL2) homology-3 (BH3) mimetics are inhibitors of protein–protein interactions (PPIs) that saturate anti-apoptotic proteins in the BCL2 family to induce apoptosis in cancer cells. Despite the success of the BH3-mimetic ABT-199 for the treatment of haematological malignancies, only a fraction of patients respond to the drug and most patients eventually develop resistance to it. Here we show that the efficacy of ABT-199 can be predicted by profiling the rewired status of the PPI network of the BCL2 family via single-molecule pull-down and co-immunoprecipitation to quantify more than 20 types of PPI from a total of only 1.2 × 106 cells per sample. By comparing the obtained multidimensional data with BH3-mimetic efficacies determined ex vivo, we constructed a model for predicting the efficacy of ABT-199 that designates two complexes of the BCL2 protein family as the primary mediators of drug effectiveness and resistance, and applied it to prospectively assist therapeutic decision-making for patients with acute myeloid leukaemia. The characterization of PPI complexes in clinical specimens opens up opportunities for individualized protein-complex-targeting therapies. The efficacy of a B-cell-lymphoma-2-homology-3 mimetic can be predicted by profiling the rewired status of the network of interactions among proteins of the B-cell-lymphoma-2 family via single-molecule pull-down and co-immunoprecipitation.
剖析蛋白质与蛋白质之间的相互作用,预测B细胞淋巴瘤-2-同源物-3模拟物对急性髓性白血病的疗效。
B细胞淋巴瘤-2(BCL2)同源-3(BH3)模拟物是一种蛋白-蛋白相互作用(PPI)抑制剂,可使BCL2家族中的抗凋亡蛋白饱和,从而诱导癌细胞凋亡。尽管BH3模拟物ABT-199在治疗血液恶性肿瘤方面取得了成功,但只有一小部分患者对这种药物有反应,而且大多数患者最终会产生耐药性。在这里,我们展示了 ABT-199 的疗效,通过单分子牵引和共沉淀技术对 BCL2 家族 PPI 网络的重联状态进行剖析,可以预测 ABT-199 的疗效。通过将获得的多维数据与体内外测定的BH3模拟药效进行比较,我们构建了一个用于预测ABT-199药效的模型,该模型将BCL2蛋白家族的两种复合物指定为药效和耐药性的主要介质,并将其应用于急性髓性白血病患者的前瞻性辅助治疗决策。临床标本中PPI复合物的特征描述为个体化蛋白质复合物靶向疗法提供了机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


