Gα13 restricts nutrient driven proliferation in mucosal germinal centers
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
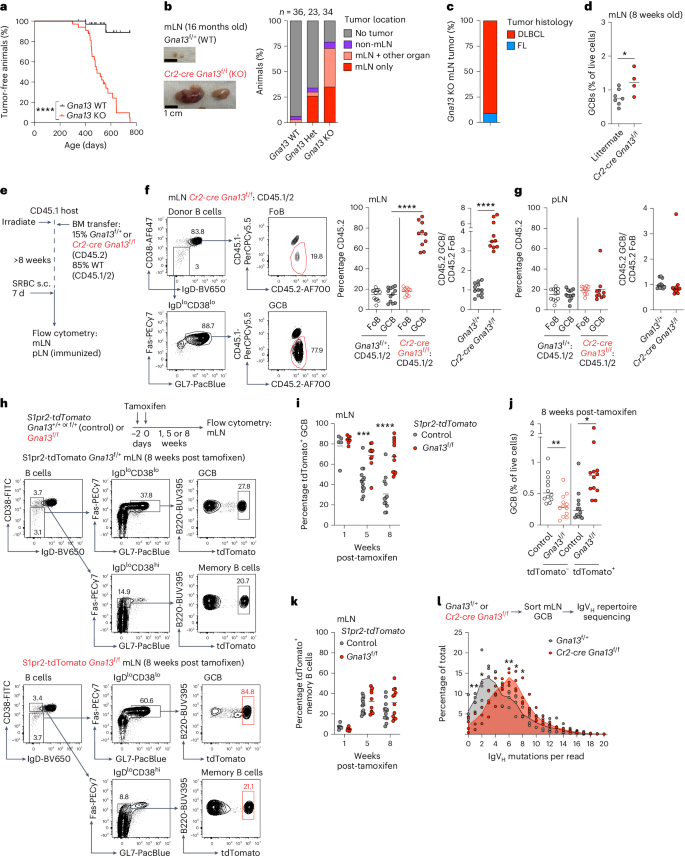
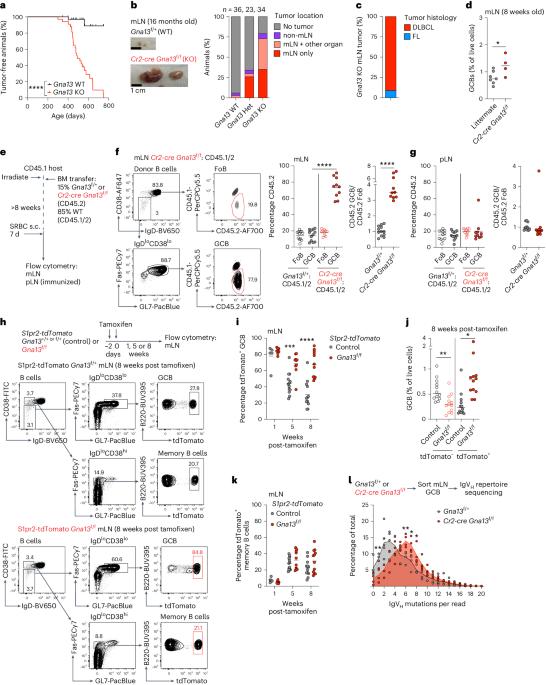
Germinal centers (GCs) that form in mucosal sites are exposed to gut-derived factors that have the potential to influence homeostasis independent of antigen receptor-driven selective processes. The G-protein Gα13 confines B cells to the GC and limits the development of GC-derived lymphoma. We discovered that Gα13-deficiency fuels the GC reaction via increased mTORC1 signaling and Myc protein expression specifically in the mesenteric lymph node (mLN). The competitive advantage of Gα13-deficient GC B cells (GCBs) in mLN was not dependent on T cell help or gut microbiota. Instead, Gα13-deficient GCBs were selectively dependent on dietary nutrients likely due to greater access to gut lymphatics. Specifically, we found that diet-derived glutamine supported proliferation and Myc expression in Gα13-deficient GCBs in the mLN. Thus, GC confinement limits the effects of dietary glutamine on GC dynamics in mucosal tissues. Gα13 pathway mutations coopt these processes to promote the gut tropism of aggressive lymphoma. Muppidi and colleagues show that loss of Gα13 drives B cell lymphomas preferentially in the mesenteric lymph nodes. They find that Gα13 is required to counteract mTORC1 and Myc signaling that is driven by the availability of dietary glutamine.
Gα13 限制了粘膜生殖中心的营养驱动增殖。
在粘膜部位形成的生殖中心(GC)会接触到肠道衍生因子,这些因子有可能影响体内平衡,而不依赖于抗原受体驱动的选择过程。G蛋白Gα13将B细胞限制在GC中,并限制GC衍生淋巴瘤的发展。我们发现,Gα13缺陷通过增加肠系膜淋巴结(mLN)中的mTORC1信号传导和Myc蛋白表达来促进GC反应。Gα13缺陷型GC B细胞(GCBs)在肠系膜淋巴结中的竞争优势并不依赖于T细胞的帮助或肠道微生物群。相反,Gα13缺陷型GCB选择性地依赖于膳食营养,这可能是因为它们有更多机会进入肠道淋巴管。具体来说,我们发现源自膳食的谷氨酰胺支持 Gα13 缺陷 GCB 在 mLN 中的增殖和 Myc 表达。因此,GC 封闭限制了膳食谷氨酰胺对粘膜组织中 GC 动态的影响。Gα13通路突变会加剧这些过程,从而促进侵袭性淋巴瘤的肠道趋向性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


