Fate of Nanobubbles Generated from CO2–Hydrate Dissociation: Coexistence with Nanodroplets—A Combined Investigation from Experiment and Molecular Dynamics Simulations
引用次数: 0
Abstract
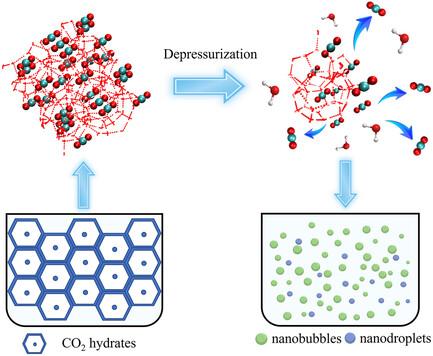
The evolution of CO2 nanobubbles generated by gas–hydrate dissociation is comprehensively studied in this research, employing a synergistic approach that combines laboratory experiments and molecular dynamics simulations. The results show that a higher concentration of nanobubbles can be observed in the early stages of hydrate dissociation, while smaller, thus-generated, nanobubbles are less stable and prefer to amalgamate into larger bubbles through coalescence or Ostwald ripening. From the high Laplace pressure inside some nanobubbles as well as their higher local densities, they may transform into nanodroplets by densification fluctuations. Thus, the dynamic coexistence of nanobubbles and -droplets is confirmed from both experimental and simulation measurements. The number and size of the nanobubbles in the system affects the interaction between water molecules and their movements so that the water molecules diffuse faster upon this condition. The water–water interactions become more pronounced in the presence of nanobubbles and the hydrogen bond network is better preserved in the bulk. This study provides new insights into the microscale mechanisms of gas–hydrate dissociation and highlights the complex interactions between nanobubbles/ -droplets, and the aqueous environment after CO2–hydrate dissociation.
二氧化碳-水合物解离产生的纳米气泡的命运:与纳米水滴共存--来自实验和分子动力学模拟的综合研究
本研究采用实验室实验和分子动力学模拟相结合的协同方法,全面研究了气体-水合物解离产生的二氧化碳纳米气泡的演变过程。结果表明,在水合物解离的早期阶段可以观察到较高浓度的纳米气泡,而由此产生的较小的纳米气泡稳定性较差,更倾向于通过凝聚或奥斯特瓦尔德熟化合并成较大的气泡。一些纳米气泡内部的拉普拉斯压力较高,局部密度也较大,因此可能会通过密度波动转化为纳米液滴。因此,实验和模拟测量都证实了纳米气泡和-液滴的动态共存。系统中纳米气泡的数量和大小会影响水分子之间的相互作用及其运动,从而使水分子在此条件下扩散得更快。在有纳米气泡存在的情况下,水与水之间的相互作用变得更加明显,氢键网络在大体积中得到了更好的保存。这项研究为气水解离的微观机制提供了新的见解,并突出了二氧化碳-水解离后纳米气泡/液滴与水环境之间复杂的相互作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


