Rh-catalysed enantioselective [2+2+1] cycloaddition reactions using three different 2π-components
IF 20
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
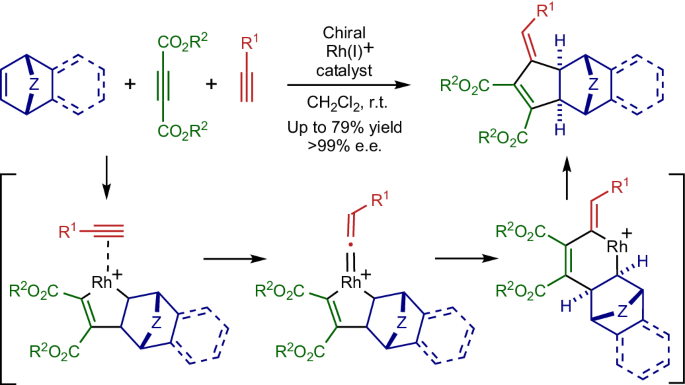
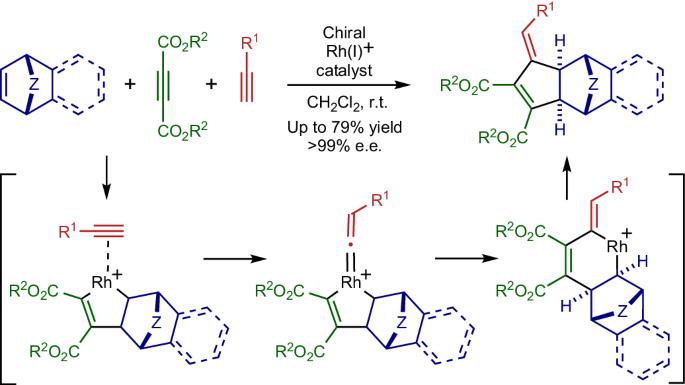
Catalytic intermolecular [2+2+2] cycloaddition reactions of three 2π-components, such as alkynes and alkenes, are a valuable method to synthesize multi-substituted six-membered carbocycles in a single step with high atom economy. When one 2π-component reacts as a one-carbon unit, a five-membered carbocycle is accessible instead of a six-membered one. However, examples of catalytic intermolecular [2+2+1] cycloaddition reactions are scarce, and enantioselective or three different 2π-component versions remain elusive. Here we report the development of a Rh-catalysed enantioselective [2+2+1] cycloaddition reaction using three different 2π-components, cycloalkenes, acetylenecarboxylates and terminal alkynes, which can form a broad range of synthetically valuable chiral 3-methylenecyclopent-1-ene derivatives with excellent selectivity. Interestingly, the three-component cycloadducts are obtained in high selectivity, while all three 2π-components are mutually reactive. Experimental and theoretical mechanistic studies reveal that the reaction proceeds via the kinetically favoured vinylidene formation from a rhodacyclopentene and a terminal alkyne. Intermolecular [2+2+1] cycloaddition reactions are challenging owing to issues with chemoselectivity. Now a Rh-catalysed enantioselective [2+2+1] cycloaddition reaction using three different 2π-components is reported. The process can form a broad range of synthetically valuable chiral 3-methylenecyclopent-1-ene derivatives with excellent selectivity.
使用三种不同 2π 成分的 Rh 催化对映体选择性 [2+2+1] 环加成反应
炔烃和烯烃等三个 2π 组份的催化分子间[2+2+2]环加成反应是一步合成多取代六元碳环的重要方法,具有很高的原子经济性。当一个 2π 组分作为单碳单元发生反应时,可获得五元碳环而不是六元碳环。然而,分子间[2+2+1]环加成反应的催化实例很少,对映选择性或三种不同的 2π 组分版本仍然难以找到。在此,我们报告了利用三种不同的 2π 组分(环烯烃、乙炔羧酸酯和端炔烃)开发的 Rh 催化的对映体选择性 [2+2+1] 环加成反应,该反应可以生成多种具有极佳选择性的、有合成价值的手性 3-亚甲基环戊-1-烯衍生物。有趣的是,三组份环加载产物的选择性很高,而所有三个 2π 组份都是相互反应的。实验和理论机理研究表明,该反应是通过斜交环戊烯和末端炔烃在动力学上有利的亚乙烯基形成进行的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


