NIR driven catalytic enhanced acute lung injury therapy by using polydopamine@Co nanozyme via scavenging ROS
IF 9.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Acute lung injury (ALI) was characterized by excessive reactive oxygen species (ROS) levels and inflammatory response in the lung. Scavenging ROS could inhibit the excessive inflammatory response, further treating ALI. Herein, we designed a novel nanozyme (P@Co) comprised of polydopamine (PDA) nanoparticles (NPs) loading with ultra-small Co, combining with near infrared (NIR) irradiation, which could efficiently scavenge intracellular ROS and suppress inflammatory responses against ALI. For lipopolysaccharide (LPS) induced macrophages, P@Co + NIR presented excellent antioxidant and anti-inflammatory capacities through lowering intracellular ROS levels, decreasing the expression levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) as well as inducing macrophage M2 directional polarization. Significantly, it displayed the outstanding activities of lowering acute lung inflammation, relieving diffuse alveolar damage, and up-regulating heat shock protein 70 (HSP70) expression, resulting in synergistic enhanced ALI therapy effect. It offers a novel strategy for the clinical treatment of ROS related diseases.
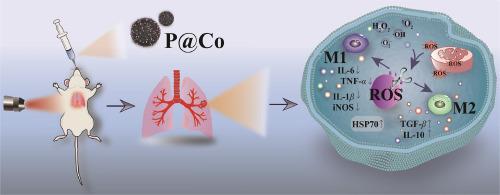
利用多巴胺@Co 纳米酶清除 ROS,在近红外驱动下催化增强急性肺损伤疗法
急性肺损伤(ALI)的特点是活性氧(ROS)水平过高和肺部炎症反应。清除 ROS 可抑制过度的炎症反应,从而进一步治疗 ALI。在此,我们设计了一种新型纳米酶(P@Co),它由负载超小型 Co 的聚多巴胺(PDA)纳米颗粒(NPs)组成,结合近红外(NIR)照射,可有效清除细胞内的 ROS 并抑制 ALI 的炎症反应。对于脂多糖(LPS)诱导的巨噬细胞,P@Co + 近红外通过降低细胞内 ROS 水平、减少白细胞介素-6(IL-6)和肿瘤坏死因子-α(TNF-α)的表达水平以及诱导巨噬细胞 M2 定向极化,表现出卓越的抗氧化和抗炎能力。值得注意的是,它在降低急性肺部炎症、缓解肺泡弥漫性损伤、上调热休克蛋白 70 (HSP70) 表达等方面表现出突出的活性,从而协同增强了 ALI 治疗效果。它为 ROS 相关疾病的临床治疗提供了一种新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


