Operando-reconstructed polyatomic ion layers boost the activity and stability of industrial current–density water splitting
IF 18.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Metal–organic frameworks have garnered attention as highly efficient pre-electrocatalysts for the oxygen evolution reaction (OER). Current structure–activity relationships primarily rely on the assumption that the complete dissolution of organic ligands occurs during electrocatalysis. Herein, modeling based on NiFe Prussian blue analogs (NiFe-PBAs) show that cyanide ligands leach from the matrix and subsequently oxidize to corresponding inorganic ions (ammonium and carbonate) that re-adsorb onto the surface of NiFe OOH during the OER process. Interestingly, the surface-adsorbed inorganic ions induce the OER reaction of NiFe OOH to switch from the adsorbate evolution to the lattice-oxygen–mediated mechanism, thus contributing to the high activity. In addition, this reconstructed inorganic ion layer acting as a versatile protective layer can prevent the dissolution of metal sites to maintain contact between catalytic sites and reactive ions, thus breaking the activity–stability trade-off. Consequently, our constructed NiFe-PBAs exhibit excellent durability for 1250 h with an ultralow overpotential of 253 mV at 100 mA cm−2. The scale-up NiFe-PBAs operated with a low energy consumption of ∼4.18 kWh m−3 H2 in industrial water electrolysis equipment. The economic analysis of the entire life cycle demonstrates that this green hydrogen production is priced at US$2.59 , meeting global targets (<US$2.5 ).
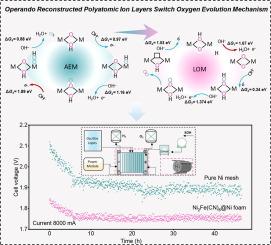
操作重建的多原子离子层提高了工业电流密度水分离的活性和稳定性
金属有机框架作为氧进化反应(OER)的高效前电催化剂备受关注。目前的结构-活性关系主要依赖于电催化过程中有机配体完全溶解的假设。在此,基于镍铁普鲁士蓝类似物(NiFe-PBAs)的建模表明,氰配体从基体中渗出,随后氧化成相应的无机离子(铵和碳酸盐),在 OER 过程中重新吸附到镍铁氧体表面。有趣的是,表面吸附的无机离子诱导 NiFe OOH 的 OER 反应从吸附剂演化机制转向晶格氧介导机制,从而提高了活性。此外,这种重构的无机离子层作为一种多功能保护层,可以防止金属位点的溶解,保持催化位点与活性离子之间的接触,从而打破活性-稳定性的权衡。因此,我们构建的 NiFe-PBA 在 100 mA cm-2 条件下具有 253 mV 的超低过电位,可持续 1250 小时。放大的 NiFe-PBA 在工业用水电解设备中的运行能耗较低,仅为 4.18 kWh m-3 H2。对整个生命周期的经济分析表明,这种绿色制氢的价格为 2.59 美元 kg-1H2,达到了全球目标(<2.5 美元 kg-1H2)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Bulletin
MULTIDISCIPLINARY SCIENCES-
CiteScore
24.60
自引率
2.10%
发文量
8092
期刊介绍:
Science Bulletin (Sci. Bull., formerly known as Chinese Science Bulletin) is a multidisciplinary academic journal supervised by the Chinese Academy of Sciences (CAS) and co-sponsored by the CAS and the National Natural Science Foundation of China (NSFC). Sci. Bull. is a semi-monthly international journal publishing high-caliber peer-reviewed research on a broad range of natural sciences and high-tech fields on the basis of its originality, scientific significance and whether it is of general interest. In addition, we are committed to serving the scientific community with immediate, authoritative news and valuable insights into upcoming trends around the globe.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


