Effect of dimethyl fumarate in patients with plaque psoriasis meeting the upgrade criteria required for moderate-to-severe disease classification
Abstract
Background
Oral dimethyl fumarate (DMF) is indicated in patients with plaque psoriasis requiring systemic treatment, including those meeting the upgrade criteria for moderate-to-severe disease classification.
Objectives
To evaluate quality of life (QoL), effectiveness, and tolerability of DMF during routine clinical practice in a patient population with plaque psoriasis meeting the upgrade criteria for moderate-to-severe disease.
Methods
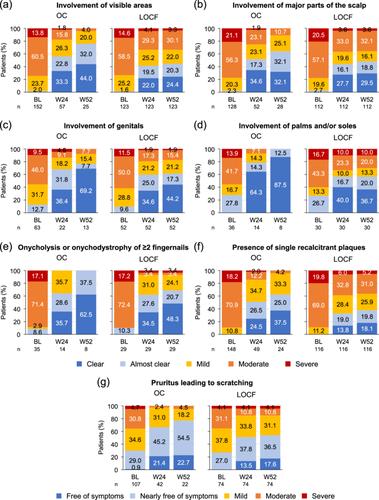
UPSKIL was a prospective, noninterventional observational study conducted in Germany from July 2019 to February 2022. Adult patients with Psoriasis Area and Severity Index (PASI) and body surface area (BSA) values ≤ 10 meeting the upgrade criteria for moderate-to-severe psoriasis were included, if therapy with DMF was indicated. Overall and by upgrade criterion, effectiveness was evaluated by change in PASI, BSA, Dermatology Life Quality Index (DLQI), Physician Global Assessment (PGA) and ItchyQoL from baseline to Weeks 24 and 52.
Results
In total, 180 patients (mean age 43.4 years, 60.3% male) were included in the effectiveness analysis. At baseline, the majority of patients suffered from involvement of visible areas (86.1%), presence of single recalcitrant plaques (83.9%), pruritus leading to scratching (81.1%) and involvement of major parts of the scalp (71.1%). Using last observation carried forward (LOCF), the proportion of patients achieving PASI < 3 increased from 7.9% at baseline to 55.3% at Week 52, and the proportion of patients achieving DLQI ≤ 5 increased from 17.4% to 52.1%. Overall and stratified by upgrade criteria, mean PGA improvement was visible in all subgroups presenting with the respective upgrade criterion as indicated by the increasing proportion of patients achieving a PGA score of 0/1 (clear/almost clear). In total, 104 patients (51.2%) experienced at least one adverse drug reaction.
Conclusions
Overall, treatment with DMF led to substantial improvement of all upgrade criteria, even when discontinued before Week 52. Safety results were consistent with the known safety profile of DMF.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


