Facile Synthesis of Hemin Derivatives with Modulated Aggregation Behaviour and Enhanced Nitric‐Oxide Scavenging Properties as New Therapeutics for Breast Cancer
IF 3.5
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Nitric oxide (•NO) plays various pathophysiological roles in breast cancer, significantly influencing the migration of tumour cells through concentration gradients. Therefore, modulating •NO levels via selective scavenging presents a promising approach to treating aggressive •NO‐dependent cancers, such as triple‐negative breast cancer (TNBC). Hemin emerges as a potential scavenger of •NO; however, its metalloporphyrin molecules tend to aggregate in physiological solutions, which limits its biomedical applications. To address this, a modification strategy is employed to minimize aggregation and protect against physiological oxidative degradation while preserving •NO‐scavenging properties. This is achieved through a simple chemical transformation that involves hemin conjugation to aromatic residues, tyrosine, and tyramine via carbodiimide reactions. These derivatives exhibit altered electronic properties and oxidation potential compared to hemin, alongside reduced aggregation tendencies and retained •NO‐binding affinity in aqueous solutions. Furthermore, depending on the type of hemin derivative, there is an associated inhibition of TNBC cell migration. These model hemin compounds demonstrate varying •NO‐binding affinities and resistance levels to oxidative degradation and aggregation, offering insights into the design of •NO‐scavenging molecules with enhanced properties for cancer treatment.
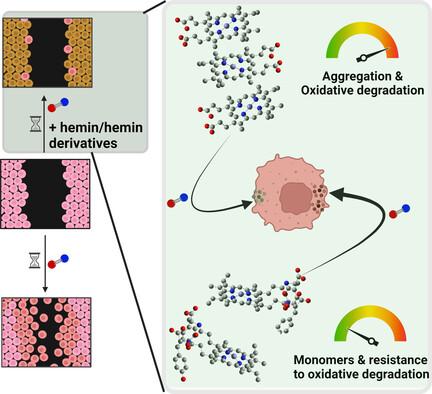
轻松合成具有调节聚集行为和增强一氧化氮清除特性的血红素衍生物,作为乳腺癌的新疗法
一氧化氮(-NO)在乳腺癌中发挥着各种病理生理作用,通过浓度梯度显著影响肿瘤细胞的迁移。因此,通过选择性清除来调节一氧化氮水平是治疗侵袭性一氧化氮依赖性癌症(如三阴性乳腺癌(TNBC))的一种很有前景的方法。血红蛋白是一种潜在的-NO清除剂,但其金属卟啉分子在生理溶液中容易聚集,这限制了其生物医学应用。为了解决这个问题,我们采用了一种修饰策略,在保持-NO 清除特性的同时,最大限度地减少聚集并防止生理氧化降解。这是通过简单的化学转化实现的,其中包括通过碳二亚胺反应将 hemin 与芳香族残基、酪氨酸和酪胺共轭。与海明相比,这些衍生物的电子特性和氧化电位都有所改变,同时在水溶液中的聚集倾向也有所降低,并保持了与 -NO 的结合亲和力。此外,根据hemin衍生物类型的不同,对TNBC细胞迁移也有相应的抑制作用。这些模型hemin化合物表现出不同的-NO结合亲和力以及抗氧化降解和聚集的能力,为设计具有增强特性的-NO清除分子用于癌症治疗提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Chemical Biology
生物-生化与分子生物学
CiteScore
7.50
自引率
5.00%
发文量
353
审稿时长
3.3 months
期刊介绍:
ACS Chemical Biology provides an international forum for the rapid communication of research that broadly embraces the interface between chemistry and biology.
The journal also serves as a forum to facilitate the communication between biologists and chemists that will translate into new research opportunities and discoveries. Results will be published in which molecular reasoning has been used to probe questions through in vitro investigations, cell biological methods, or organismic studies.
We welcome mechanistic studies on proteins, nucleic acids, sugars, lipids, and nonbiological polymers. The journal serves a large scientific community, exploring cellular function from both chemical and biological perspectives. It is understood that submitted work is based upon original results and has not been published previously.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


