A KLF2-BMPER-Smad1/5 checkpoint regulates high fluid shear stress-mediated artery remodeling
IF 9.4
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
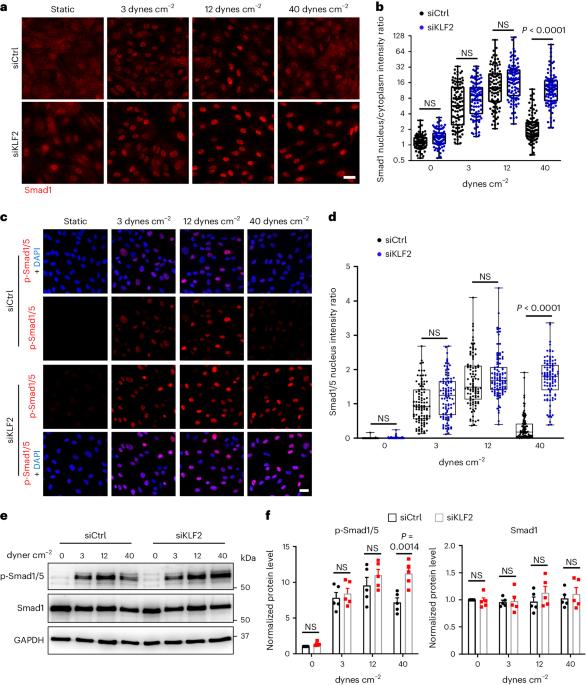
Vascular remodeling to match arterial diameter to tissue requirements commonly fails in ischemic disease. Endothelial cells sense fluid shear stress (FSS) from blood flow to maintain FSS within a narrow range in healthy vessels. Thus, high FSS induces vessel outward remodeling, but mechanisms are poorly understood. We previously reported that Smad1/5 is maximally activated at physiological FSS. Smad1/5 limits Akt activation, suggesting that inhibiting Smad1/5 may facilitate outward remodeling. Here we report that high FSS suppresses Smad1/5 by elevating KLF2, which induces the bone morphogenetic protein (BMP) pathway inhibitor, BMP-binding endothelial regulator (BMPER), thereby de-inhibiting Akt. In mice, surgically induced high FSS elevated BMPER expression, inactivated Smad1/5 and induced vessel outward remodeling. Endothelial BMPER deletion impaired blood flow recovery and vascular remodeling. Blocking endothelial cell Smad1/5 activation with BMP9/10 blocking antibodies improved vascular remodeling in mouse models of type 1 and type 2 diabetes. Suppression of Smad1/5 is thus a potential therapeutic approach for ischemic disease. Deng et al. show that endothelial cells respond to high fluid shear stress by KLF2-mediated induction of the BMP–Smad1/5 pathway inhibitor BMPER, resulting in outward vessel remodeling, and apply this knowledge to develop an approach that improves vessel remodeling in mouse models of diabetes.
KLF2-BMPER-Smad1/5检查点调节高流体剪切应力介导的动脉重塑
在缺血性疾病中,使动脉直径与组织要求相匹配的血管重塑通常会失败。内皮细胞能感知血流中的流体剪切应力(FSS),从而将健康血管中的FSS维持在一个狭窄的范围内。因此,高 FSS 会诱导血管向外重塑,但其机制尚不清楚。我们以前曾报道过,Smad1/5 在生理 FSS 时被最大限度地激活。Smad1/5 限制了 Akt 的激活,这表明抑制 Smad1/5 可能会促进血管向外重塑。在这里,我们报告了高 FSS 可通过提高 KLF2 抑制 Smad1/5,KLF2 可诱导骨形态发生蛋白(BMP)通路抑制剂 BMP 结合内皮调节因子(BMPER),从而抑制 Akt。在小鼠体内,手术诱导的高 FSS 会提高 BMPER 的表达,使 Smad1/5 失活,并诱导血管向外重塑。内皮 BMPER 缺失会损害血流恢复和血管重塑。用 BMP9/10 阻断抗体阻断内皮细胞 Smad1/5 的活化可改善 1 型和 2 型糖尿病小鼠模型的血管重塑。因此,抑制 Smad1/5 是治疗缺血性疾病的一种潜在方法。Deng 等人的研究表明,内皮细胞通过 KLF2 介导的 BMP-Smad1/5 通路抑制剂 BMPER 的诱导对高流体剪切应力做出反应,从而导致血管向外重塑。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


