Effect of Lactobacillus gasseri BIO6369 and Lacticaseibacillus rhamnosus BIO5326 on Gastric Carcinogenesis Induced by Helicobacter pylori Infection
Abstract
Background
Helicobacter pylori infection-associated gastric adenocarcinoma is influenced by various factors, including the digestive microbiota. Lactic acid bacteria role in digestive carcinogenesis has been discussed, and some Lactobacillaceae family species have been shown to act against H. pylori-induced inflammation and colonization. However, their effects on H. pylori-related carcinogenesis have not yet been studied. Lactobacillaceae family effects on the epithelial-to-mesenchymal transition (EMT), emergence of cells with cancer stem cell (CSC) properties and the pro-inflammatory response of gastric epithelial cells to H. pylori infection were investigated.
Materials and Methods
A co-culture model of AGS gastric epithelial cells infected with a carcinogenic strain of H. pylori associated with 18 different probiotic strains candidates were used. Different EMT indicators and CSC properties were studied, including quantification of the mesenchymal phenotype, tumorsphere formation, EMT marker expression, and tight junction evaluation with immunofluorescence microscopy. The effect of the strains on the pro-inflammatory response to H. pylori was also evaluated by quantifying interleukin-8 (IL-8) production using ELISA.
Results
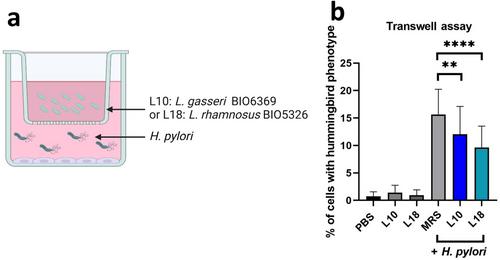
Among the strains tested, Lactobacillus gasseri BIO6369 and Lacticaseibacillus rhamnosus BIO5326 induced a 30.6% and 38.4% reduction in the mesenchymal phenotype, respectively, caused a significant decrease in Snail and Zeb1 EMT marker expression and prevented the loss of tight junctions induced by H. pylori infection. A separate co-culture with a Boyden chamber maintained the effects induced by the two strains. H. pylori-induced IL-8 production was also significantly reduced in the presence of L. gasseri BIO6369 and L. rhamnosus BIO5326.
Conclusion
Lactobacillus gasseri BIO6369 and L. rhamnosus BIO5326 strains decreased epithelial-to-mesenchymal transition and inflammation induced by H. pylori infection, suggesting that these species may have a protective effect against H. pylori-induced gastric carcinogenesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


