Differences in the effectiveness and safety of different renal denervation devices
IF 4.6
2区 医学
Q1 PERIPHERAL VASCULAR DISEASE
引用次数: 0
Abstract
Renal denervation (RDN) is a minimally invasive, endovascular catheter-based procedure using radiofrequency, ultrasound, or alcohol-mediated ablation to treat resistant hypertension. RDN gained popularity in 2009 when it was shown to have an antihypertensive effect. However, concerns about the efficacy of RDN were raised in the HTN-3 trial published in 2014, and the development of several RDN devices was then discontinued. In the process, new randomized controlled trials were conducted after the development of some of the RDN devices, the quality assurance of the procedure, changes in ablation points, and improvements in study design. In November 2023, the U.S. Food and Drug Administration approved a radiofrequency RDN device and an ultrasound RDN device. The results of a randomized controlled trial of an alcohol-mediated RDN device have been published, and future trends are being watched closely. In this mini-review, we summarize the differences in the antihypertensive effect and safety of the different RDN devices and the endpoints of the procedure in order to contribute to the further development of RDN devices Currently available renal denervation device. (A) multielectrode radiofrequency ablation (Spyral), (B) ultrasound denervation (Paraise), and (C) alcohol-mediated perivascular denervation (Peregrine). ASBP; ambulatory systolic blood pressure, ADBP; ambulatory diastolic blood pressure, OSBP; office systolic blood pressure, ODBP; office diastolic blood pressure. Analysis according to types of renal denervation device (radiofrequency, ultrasound, or alcohol-mediated device). P values for interaction were 0.578 (ambulatory SBP), 0.499 (ambulatory diastolic BP), 0.853 (office SBP), and 0.870 (office diastolic BP).
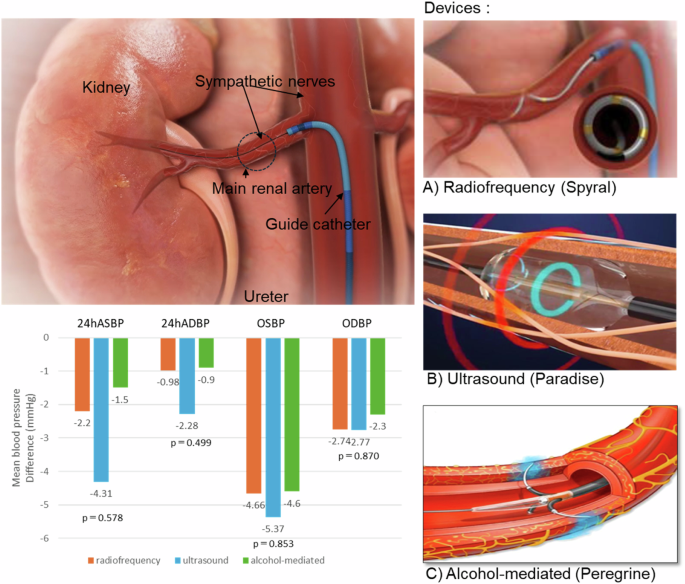

不同肾脏去势装置的有效性和安全性差异。
肾脏去神经支配(RDN)是一种基于血管内导管的微创手术,使用射频、超声或酒精介导消融来治疗抵抗性高血压。2009 年,当 RDN 被证明具有降压效果时,它开始流行起来。然而,2014 年发表的 HTN-3 试验引起了人们对 RDN 疗效的担忧,随后停止了几种 RDN 设备的开发。在这一过程中,部分 RDN 设备的开发、手术质量的保证、消融点的改变以及研究设计的改进之后,又开展了新的随机对照试验。2023 年 11 月,美国食品和药物管理局批准了一种射频 RDN 设备和一种超声 RDN 设备。酒精介导的 RDN 设备的随机对照试验结果已经公布,未来的趋势正受到密切关注。在这篇微型综述中,我们总结了不同 RDN 设备在降压效果和安全性方面的差异以及手术的终点,以便为 RDN 设备的进一步发展做出贡献 目前可用的肾脏去神经支配设备。多电极射频消融(Spyral)、(B)超声去神经支配(Paraise)和(C)酒精介导的血管周围去神经支配(Peregrine)。ASBP 动态收缩压,ADBP 动态舒张压,OSBP 办公室收缩压,ODBP 办公室舒张压。根据肾脏去神经支配装置的类型(射频、超声或酒精介导装置)进行分析。交互作用的 P 值分别为 0.578(流动性收缩压)、0.499(流动性舒张压)、0.853(办公室收缩压)和 0.870(办公室舒张压)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Hypertension Research
医学-外周血管病
CiteScore
7.40
自引率
16.70%
发文量
249
审稿时长
3-8 weeks
期刊介绍:
Hypertension Research is the official publication of the Japanese Society of Hypertension. The journal publishes papers reporting original clinical and experimental research that contribute to the advancement of knowledge in the field of hypertension and related cardiovascular diseases. The journal publishes Review Articles, Articles, Correspondence and Comments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


