Tailoring Stepped Layered Titanates for Sodium-Ion Battery Applications
IF 14.7
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
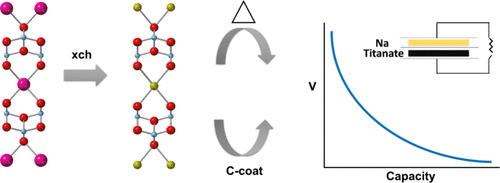
Concerns about sustainability and supply chain issues associated with lithium-ion batteries (LIBs) have led researchers and companies around the world to investigate alternative technologies. Of all the so-called “beyond LIBs”, sodium-ion batteries (NIBs) are in the most advanced stage of development, and are being considered for grid storage applications as well as moderate-range electric vehicles. While graphite is the most commonly used anode material for LIBs, hard carbons are used in NIBs because sodium insertion into graphite does not occur to a useful extent. Other possibilities, based on cost and availability arguments, are titanates, which are generally denser than disordered carbons, meaning more material can be packed into a given volume, leading potentially to greater energy density. We have researched stepped layered titanates for use as anode materials, focusing on two types of structures. The first is “sodium nonatitanate” or NNT, with the composition NaTi3O6(OH)·2H2O having six Ti4+O6 octahedra joined together in steps to form layers with sodium ions and water in-between. The lepidocrocite-type titanate structure, contains zigzag layers (or steps one Ti4+O6 unit across). These exist in a wide range of compositions, and contain large exchangeable cations between the layers. An unusual feature of both NNT and the lepidocrocite titanates is the very low potentials (0.3–0.5 V vs Na+/Na) at which they insert sodium. This makes them particularly attractive for anode applications. Another interesting feature is the ability to tailor the electrochemical properties by various modifications, such as heat-treatment to remove water and change the structure, introduction of vacancies, ion-exchange, surface modifications, and carbon coating or graphene wrapping, all of which alter the electrochemical properties. Finally, heterostructuring (interleaving titanate layers with carbon) results in new materials with different redox properties. For all the titanates, the first cycle Coulombic efficiency (C.E.) is very sensitive to the binder used in the electrode fabrication and the electrolyte used. Because sodium insertion occurs at such a low potential, some electrolyte and binder are irreversibly reduced during the first cycle to form a protective solid electrolyte interphase (SEI). In a full cell, it is important to maximize the C.E. because all the cyclable sodium must come from the cathode, so cells must be overbuilt to compensate for these losses. Proper selection of binder and electrolyte results in improved cycling performance and minimal first cycle losses. Finally, examples of full cells containing some of the materials under discussion are provided.
为钠离子电池应用定制阶梯层状钛酸盐
对锂离子电池(LIB)的可持续性和供应链问题的担忧,促使世界各地的研究人员和公司开始研究替代技术。在所有所谓的 "超越锂离子电池 "中,钠离子电池(NIB)正处于最先进的开发阶段,目前正被考虑用于电网存储应用以及中程电动汽车。石墨是锂离子电池最常用的负极材料,而硬碳则用于 NIB,因为钠插入石墨的程度并不高。基于成本和可用性的考虑,钛酸盐也是一种可能的材料,其密度通常高于无序碳,这意味着在一定体积内可以容纳更多的材料,从而有可能提高能量密度。我们对用作阳极材料的阶梯层状钛酸盐进行了研究,重点关注两种类型的结构。第一种是 "非钛酸钠 "或 NNT,其成分为 NaTi3O6(OH)-2H2O,有六个 Ti4+O6 八面体以阶梯状连接在一起,形成夹有钠离子和水的层。鳞片闪石型钛酸酯结构包含 "之 "字形层(或横跨一个 Ti4+O6 单元的阶梯)。这些结构的组成范围很广,层与层之间含有大量可交换阳离子。NNT 和鳞片钛酸盐的一个不同寻常之处在于,它们插入钠的电位非常低(0.3-0.5 V vs Na+/Na)。这使它们在阳极应用中特别具有吸引力。另一个有趣的特点是可以通过各种改性来定制电化学特性,例如通过热处理去除水分并改变结构、引入空位、离子交换、表面改性、碳涂层或石墨烯包裹,所有这些都会改变电化学特性。最后,异质结构(钛酸酯层与碳层交错)会产生具有不同氧化还原特性的新材料。对于所有钛酸盐来说,第一周期库仑效率(C.E.)对电极制造中使用的粘合剂和电解液非常敏感。由于钠插入是在如此低的电位下发生的,因此在第一个循环期间,一些电解质和粘结剂会发生不可逆的还原,形成保护性的固体电解质间相(SEI)。在全电池中,由于所有可循环的钠都必须来自阴极,因此必须最大限度地提高 C.E.,因此电池必须过载以补偿这些损失。正确选择粘合剂和电解液可提高循环性能,并将第一循环损耗降至最低。最后,还提供了包含所讨论的某些材料的完整电池实例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


