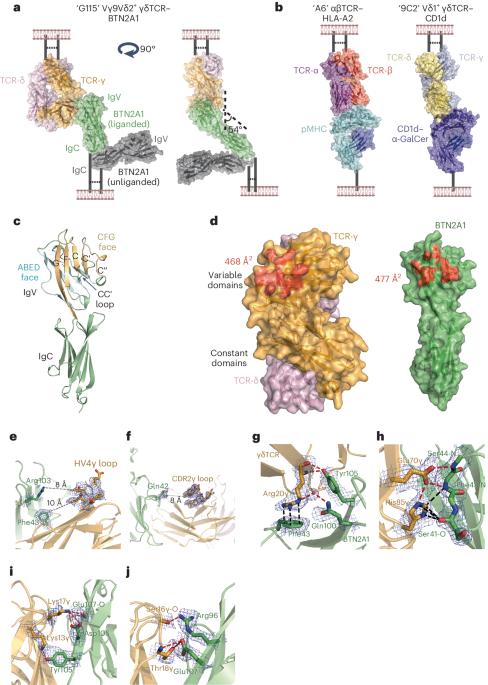
Vγ9Vδ2 T cells recognize butyrophilin 2A1 and 3A1 heteromers
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Butyrophilin (BTN) molecules are emerging as key regulators of T cell immunity; however, how they trigger cell-mediated responses is poorly understood. Here, the crystal structure of a gamma-delta T cell antigen receptor (γδTCR) in complex with BTN2A1 revealed that BTN2A1 engages the side of the γδTCR, leaving the apical TCR surface bioavailable. We reveal that a second γδTCR ligand co-engages γδTCR via binding to this accessible apical surface in a BTN3A1-dependent manner. BTN2A1 and BTN3A1 also directly interact with each other in cis, and structural analysis revealed formation of W-shaped heteromeric multimers. This BTN2A1–BTN3A1 interaction involved the same epitopes that BTN2A1 and BTN3A1 each use to mediate the γδTCR interaction; indeed, locking BTN2A1 and BTN3A1 together abrogated their interaction with γδTCR, supporting a model wherein the two γδTCR ligand-binding sites depend on accessibility to cryptic BTN epitopes. Our findings reveal a new paradigm in immune activation, whereby γδTCRs sense dual epitopes on BTN complexes. In this study, Uldrich and colleagues describe the crystal structure of the Vγ9Vδ2 T cell antigen receptor (TCR) interacting with BTN2A1 and demonstrate the existence of a second ligand that co-binds to a distinct epitope on Vγ9Vδ2 TCR. Using these data, the authors suggest a model of Vγ9Vδ2 TCR activation in which BTN2A1 and BTN3A1 are tethered to each other at the steady state, and must disengage to allow TCR binding.
Vγ9Vδ2 T 细胞识别丁淀粉样蛋白 2A1 和 3A1 异构体
嗜丁蛋白(BTN)分子正在成为 T 细胞免疫的关键调节因子;然而,人们对它们如何触发细胞介导的反应却知之甚少。在这里,γ-δ T 细胞抗原受体(γδTCR)与 BTN2A1 复合物的晶体结构显示,BTN2A1 与γδTCR 的一侧接合,使顶端 TCR 表面具有生物可利用性。我们发现,第二种γδTCR 配体以一种依赖于 BTN3A1 的方式通过与这一可利用的顶端表面结合来共同参与γδTCR。BTN2A1 和 BTN3A1 还直接以顺式相互作用,结构分析表明它们形成了 W 型异构多聚体。这种 BTN2A1-BTN3A1 相互作用涉及 BTN2A1 和 BTN3A1 各自用来介导γδTCR 相互作用的相同表位;事实上,将 BTN2A1 和 BTN3A1 锁定在一起会减弱它们与γδTCR 的相互作用,这支持了一种模型,即两个γδTCR 配体结合位点取决于对隐性 BTN 表位的可及性。我们的研究结果揭示了一种新的免疫激活模式,即γδTCR 可感知 BTN 复合物上的双重表位。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


