Skeletal muscle p53-depletion uncovers a mechanism of fuel usage suppression that enables efficient energy conservation
Abstract
Background
The ability of skeletal muscle to respond adequately to changes in nutrient availability, known as metabolic flexibility, is essential for the maintenance of metabolic health and loss of flexibility contributes to the development of diabetes and obesity. The tumour suppressor protein, p53, has been linked to the control of energy metabolism. We assessed its role in the acute control of nutrient allocation in skeletal muscle in the context of limited nutrient availability.
Methods
A mouse model with inducible deletion of the p53-encoding gene, Trp53, in skeletal muscle was generated using the Cre-loxP-system. A detailed analysis of nutrient metabolism in mice with control and knockout genotypes was performed under ad libitum fed and fasting conditions and in exercised mice.
Results
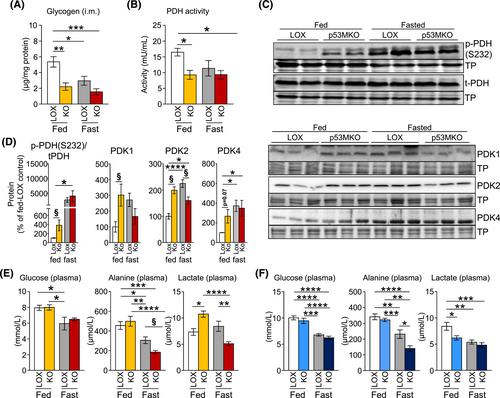
Acute deletion of p53 in myofibres of mice activated catabolic nutrient usage pathways even under ad libitum fed conditions, resulting in significantly increased overall energy expenditure (+10.6%; P = 0.0385) and a severe nutrient deficit in muscle characterized by depleted intramuscular glucose and glycogen levels (−62,0%; P < 0.0001 and −52.7%; P < 0.0001, respectively). This was accompanied by changes in marker gene expression patterns of circadian rhythmicity and hyperactivity (+57.4%; P = 0.0068). These metabolic changes occurred acutely, within 2–3 days after deletion of Trp53 was initiated, suggesting a rapid adaptive response to loss of p53, which resulted in a transient increase in lactate release to the circulation (+46.6%; P = 0.0115) from non-exercised muscle as a result of elevated carbohydrate mobilization. Conversely, an impairment of proteostasis and amino acid metabolism was observed in knockout mice during fasting. During endurance exercise testing, mice with acute, muscle-specific Trp53 inactivation displayed an early exhaustion phenotype with a premature shift in fuel usage and reductions in multiple performance parameters, including a significantly reduced running time and distance (−13.8%; P = 0.049 and −22.2%; P = 0.0384, respectively).
Conclusions
These findings suggest that efficient nutrient conservation is a key element of normal metabolic homeostasis that is sustained by p53. The homeostatic state in metabolic tissues is actively maintained to coordinate efficient energy conservation and metabolic flexibility towards nutrient stress. The acute deletion of Trp53 unlocks mechanisms that suppress the activity of nutrient catabolic pathways, causing substantial loss of intramuscular energy stores, which contributes to a fasting-like state in muscle tissue. Altogether, these findings uncover a novel function of p53 in the short-term regulation of nutrient metabolism in skeletal muscle and show that p53 serves to maintain metabolic homeostasis and efficient energy conservation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


