Diastereoselective Synthesis of Cyclopenta[c]chromanones via Pd0-Catalyzed [3+2] Cycloaddition.
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-08-02
Epub Date: 2024-07-16
DOI:10.1021/acs.joc.4c00836
引用次数: 0
Abstract
A straightforward method for synthesizing cyclopenta[c]chromanones is demonstrated. The strategy involves a [3+2] cycloaddition of VCPs and activated coumarins under Pd-catalyzed conditions. The reaction provides the desired products bearing up to four consecutive stereocenters with high diastereocontrol. Control experiments and density functional theory studies support the proposed mechanism and the observed diastereoselectivity in the product through a thermodynamically controlled pathway.
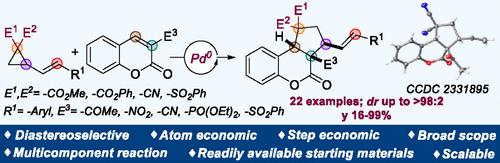
通过 Pd0 催化 [3+2] 环加成非对映选择性合成环戊并[c]色满酮。
本文展示了一种合成环戊并[c]色满酮的直接方法。该方法涉及 VCP 与活化香豆素在 Pd 催化条件下进行 [3+2] 环加成反应。该反应以高度的非对映控制提供了含有多达四个连续立体中心的所需产物。控制实验和密度泛函理论研究支持了所提出的机理,并通过热力学控制途径观察到了产物的非对映选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


