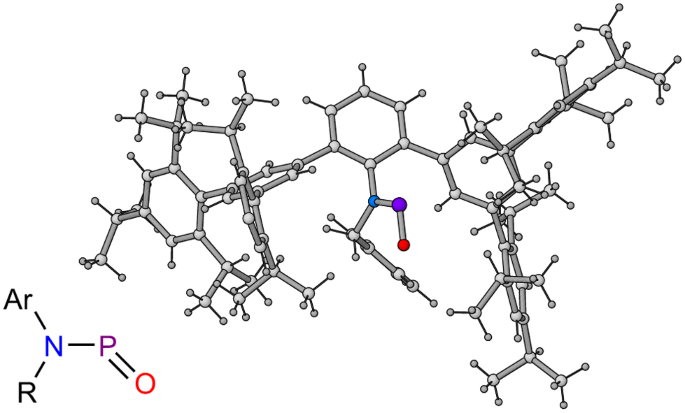
Isolation and characterization of a two-coordinate phosphinidene oxide
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Nitroso compounds, R–N=O, are common intermediates in organic synthesis, and are typically amenable to storage and manipulation at ambient temperature under aerobic conditions. By contrast, phosphorus-containing analogues, such as R–P=O (R = OH, CH3, OCH3, Ph), are extremely reactive and need to be studied in inert gas matrices at ultralow temperatures (3–15 K). These species are believed to be key intermediates in the degradation/combustion of organic phosphorus compounds, a class of chemicals that includes chemical warfare agents and flame retardants. Here we describe the isolation of a two-coordinate phosphorus(III) oxide under ambient conditions, enabled by the use of an extremely bulky amine ligand. Reactivity studies reveal that the phosphorus centre can be readily oxidized, and that in doing so, the P–O bond remains intact, an observation that is of interest to the proposed reactivity of transient phosphorus(III) oxides. Phosphinidene oxides are intermediates in the combustion of organic phosphorus compounds; however, they are highly unstable and their observation requires ultralow temperatures. Now it has been shown that a combination of steric bulk and electronic stabilization enables the isolation and manipulation of a two-coordinate phosphorus(III) oxide compound at room temperature.
双配位氧化膦的分离与表征
亚硝基化合物(R-N=O)是有机合成中常见的中间体,通常适合在有氧条件下的环境温度下储存和操作。相比之下,含磷类似物,如 R-P=O(R = OH、CH3、OCH3、Ph),反应性极强,需要在超低温(3-15 K)的惰性气体基质中进行研究。这些物种被认为是有机磷化合物降解/燃烧过程中的关键中间体,这一类化学物质包括化学战剂和阻燃剂。在这里,我们描述了在环境条件下,通过使用一种极其笨重的胺配体,分离出一种双配位磷(III)氧化物。反应性研究表明,磷中心很容易被氧化,而在氧化过程中,P-O 键保持完好无损,这一观察结果与所提出的瞬态磷(III)氧化物的反应性有关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


