Heterotrimeric collagen helix with high specificity of assembly results in a rapid rate of folding
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The most abundant natural collagens form heterotrimeric triple helices. Synthetic mimics of collagen heterotrimers have been found to fold slowly, even compared to the already slow rates of homotrimeric helices. These prolonged folding rates are not understood. Here we compare the stabilities, specificities and folding rates of three heterotrimeric collagen mimics designed through a computationally assisted approach. The crystal structure of one ABC-type heterotrimer verified a well-controlled composition and register and elucidated the geometry of pairwise cation–π and axial and lateral salt bridges in the assembly. This collagen heterotrimer folds much faster (hours versus days) than comparable, well-designed systems. Circular dichroism and NMR data suggest the folding is frustrated by unproductive, competing heterotrimer species and these species must unwind before refolding into the thermodynamically favoured assembly. The heterotrimeric collagen folding rate is inhibited by the introduction of preformed competing triple-helical assemblies, which suggests that slow heterotrimer folding kinetics are dominated by the frustration of the energy landscape caused by competing triple helices. The mechanism of collagen heterotrimer folding is difficult to recapitulate synthetically. Now an ABC collagen mimetic heterotrimer has been designed that employs pairwise amino acid interactions, validated by X-ray crystallography, to promote composition- and register-specific assembly. The high specificity of its assembly leads to an increased rate of folding compared with similar collagen heterotrimers.
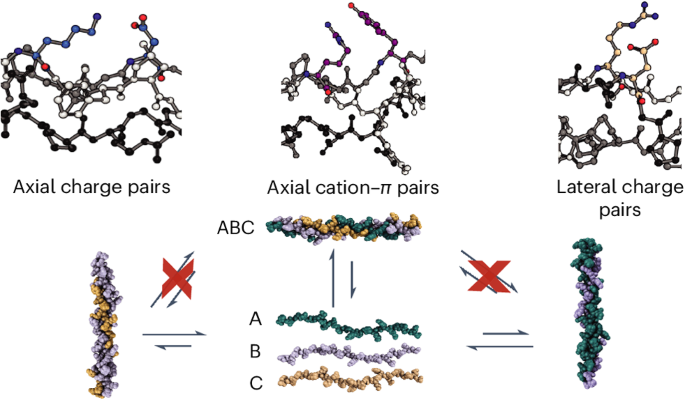
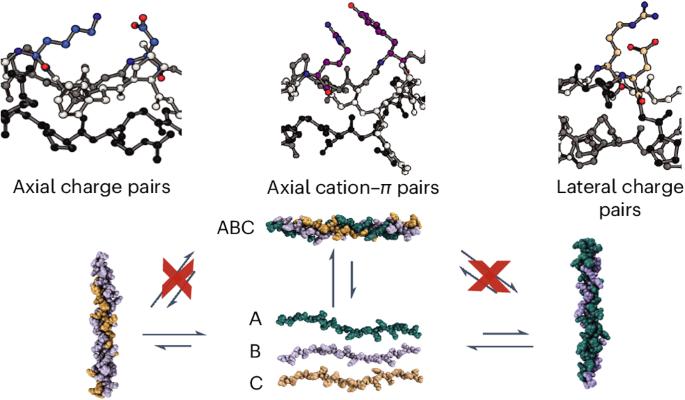
具有高度装配特异性的异三聚胶原蛋白螺旋可实现快速折叠
最丰富的天然胶原蛋白形成异三聚体三螺旋。研究发现,胶原异三聚体的合成模拟物折叠速度很慢,甚至比同三聚体螺旋的折叠速度还要慢。人们对这些延长的折叠速度尚不了解。在这里,我们比较了通过计算辅助方法设计的三种异三聚体胶原蛋白模拟物的稳定性、特异性和折叠率。其中一个 ABC 型异源三聚体的晶体结构验证了其组成和寄存器的良好控制,并阐明了组装中成对的阳离子π和轴向及横向盐桥的几何形状。这种胶原异源三聚体的折叠速度(数小时而非数天)远远快于设计良好的同类系统。环二色性和核磁共振数据表明,折叠受到了非生产性、竞争性异源三聚体物种的阻碍,这些物种必须在重新折叠成热力学上有利的组装体之前解开。异三聚体胶原蛋白的折叠速率受到预先形成的竞争性三螺旋装配的抑制,这表明缓慢的异三聚体折叠动力学是由竞争性三螺旋造成的能量景观受挫主导的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


