Mitochondrial bioenergetics are not associated with myofibrillar protein synthesis rates
Abstract
Background
Mitochondria represent key organelles influencing cellular homeostasis and have been implicated in the signalling events regulating protein synthesis.
Methods
We examined whether mitochondrial bioenergetics (oxidative phosphorylation and reactive oxygen species (H2O2) emission, ROS) measured in vitro in permeabilized muscle fibres represent regulatory factors for integrated daily muscle protein synthesis rates and skeletal muscle mass changes across the spectrum of physical activity, including free-living and bed-rest conditions: n = 19 healthy, young men (26 ± 4 years, 23.4 ± 3.3 kg/m2) and following 12 weeks of resistance-type exercise training: n = 10 healthy older men (70 ± 3 years, 25.2 ± 2.1 kg/m2). Additionally, we evaluated the direct relationship between attenuated mitochondrial ROS emission and integrated daily myofibrillar and sarcoplasmic protein synthesis rates in genetically modified mice (mitochondrial-targeted catalase, MCAT).
Results
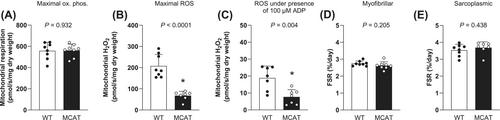
Neither oxidative phosphorylation nor H2O2 emission were associated with muscle protein synthesis rates in healthy young men under free-living conditions or following 1 week of bed rest (both P > 0.05). Greater increases in GSSG concentration were associated with greater skeletal muscle mass loss following bed rest (r = −0.49, P < 0.05). In older men, only submaximal mitochondrial oxidative phosphorylation (corrected for mitochondrial content) was positively associated with myofibrillar protein synthesis rates during exercise training (r = 0.72, P < 0.05). However, changes in oxidative phosphorylation and H2O2 emission were not associated with changes in skeletal muscle mass following training (both P > 0.05). Additionally, MCAT mice displayed no differences in myofibrillar (2.62 ± 0.22 vs. 2.75 ± 0.15%/day) and sarcoplasmic (3.68 ± 0.35 vs. 3.54 ± 0.35%/day) protein synthesis rates when compared with wild-type mice (both P > 0.05).
Conclusions
Mitochondrial oxidative phosphorylation and reactive oxygen emission do not seem to represent key factors regulating muscle protein synthesis or muscle mass regulation across the spectrum of physical activity.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


