Copper-catalyzed asymmetric 1,2-arylboration of enamines: access to chiral borate-containing 3,3′-disubstituted isoindolinones†
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An enantioselective copper-catalyzed 1,2-arylboration reaction of enamines has been developed by employing (R)-xyl-BINAP as a chiral ligand. A number of chiral borate-containing 3,3′-disubstituted isoindolinones were obtained in moderate to good yields and good to excellent enantioselectivities from the reactions of N-(o-iodobenzoyl)enamines and bis(pinacolato)diboron (B2pin2) under mild reaction conditions. Synthetic transformations of the products were conducted to demonstrate the practicality of this reaction.
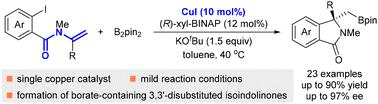
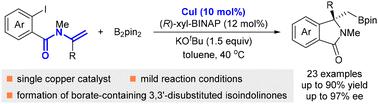
铜催化烯胺的不对称 1,2-芳基硼化反应:获得手性含硼酸盐的 3,3'-二取代异吲哚啉酮。
采用 (R)-xyl-BINAP 作为手性配体,开发了铜催化的 1,2-芳基硼化烯胺对映体选择性反应。在温和的反应条件下,通过 N-(邻碘苯甲酰基)烯胺和双(频哪酮)二硼酸酯(B2pin2)的反应,获得了一些手性含硼酸酯的 3,3'-二取代异吲哚啉酮,产率中等至良好,对映选择性良好至极佳。为了证明该反应的实用性,对产物进行了合成转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


