Characterization of obesity-related diseases and inflammation using single cell immunophenotyping in two different diet-induced obesity models
IF 4.2
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
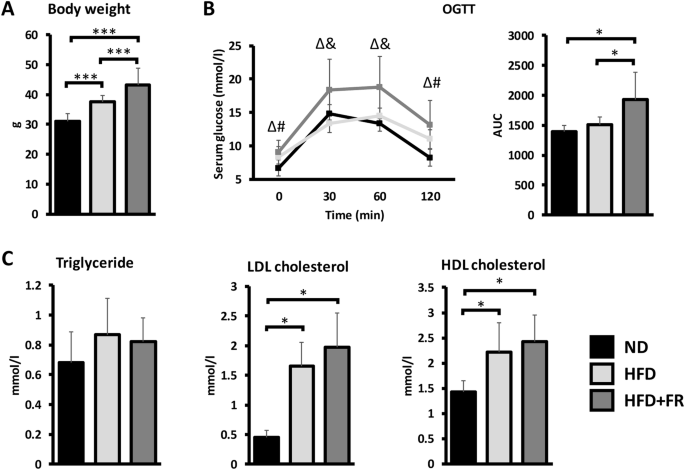
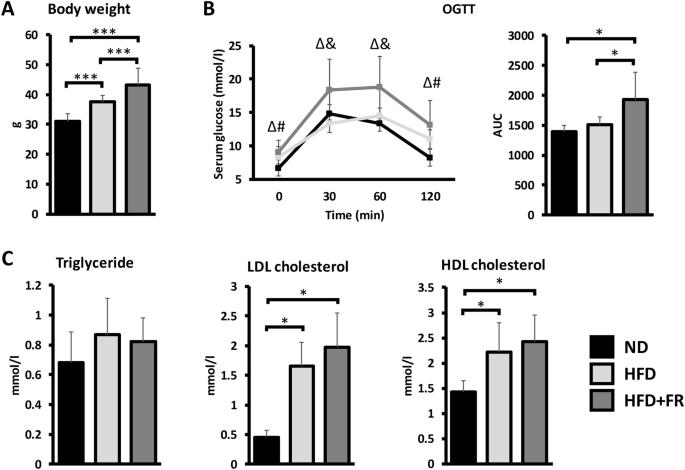
Obesity is a growing problem worldwide and a major risk factor for many chronic diseases. The accumulation of adipose tissue leads to the release of significant amounts of pro-inflammatory cytokines and adipokines, resulting in a low-grade systemic inflammation. However, the mechanisms behind the development of obesity-related diseases are not fully understood. Therefore, our study aimed to investigate the pathological changes and inflammatory processes at systemic level and in individual organs in two different diet-induced mouse obesity models. Male C57BL6/J mice were fed by high-fat diet (HFD), high-fat/high-fructose diet (HFD + FR) or normal chow for 21 weeks starting at 3 months of age (n = 15 animals/group). Insulin resistance was tested by oral glucose tolerance test. Pathological changes were investigated on hematoxylin–eosin-stained liver and brown adipose tissue sections. The gene expression levels of adipokines and cytokines were analyzed by qPCR in adipose tissues, whereas serum protein concentrations were determined by multiplex immunoassays. Immunophenotyping of isolated blood, bone marrow and spleen cells was performed by single-cell mass cytometry. Weight gain, glucose intolerance and hepatic steatosis were more severe in the HFD + FR group than in the control and HFD groups. This was accompanied by a higher level of systemic inflammation, as indicated by increased expression of pro-inflammatory genes in visceral white adipose tissue and by a higher serum TNFα level. In addition, immunophenotyping revealed the increase of the surface expressions of CD44 and CD69 on various cell types, such as CD8+ and CD4 + T-cells, B-cells and macrophages, in animals with obesity. The combination of HFD with fructose supplementation promotes more properly the symptoms of metabolic syndrome. Therefore, the combined high-fat/high-fructose nutrition can be a more suitable model of the Western diet. However, despite these differences, both models showed immunophenotypic changes that may be associated with increased risk of obesity-related cancer.
在两种不同饮食诱导的肥胖模型中利用单细胞免疫分型分析肥胖相关疾病和炎症的特征
背景肥胖是全世界日益严重的问题,也是许多慢性疾病的主要风险因素。脂肪组织的堆积会导致大量促炎细胞因子和脂肪因子的释放,从而引发低度全身性炎症。然而,肥胖相关疾病的发病机制尚未完全明了。因此,我们的研究旨在探讨两种不同饮食诱导的小鼠肥胖模型在全身水平和个别器官中的病理变化和炎症过程。方法雄性 C57BL6/J 小鼠从 3 个月大开始,连续 21 周以高脂饮食(HFD)、高脂/高果糖饮食(HFD + FR)或正常饲料喂养(n = 15 只/组)。通过口服葡萄糖耐量试验检测胰岛素抵抗。对苏木精-伊红染色的肝脏和棕色脂肪组织切片进行病理变化研究。脂肪组织中脂肪因子和细胞因子的基因表达水平通过 qPCR 进行分析,血清蛋白浓度则通过多重免疫测定法测定。结果 与对照组和高脂饮食组相比,高脂饮食 + FR 组的体重增加、葡萄糖不耐受和肝脏脂肪变性更为严重。与此同时,内脏白色脂肪组织中的促炎基因表达增加,血清 TNFα 水平升高,表明全身炎症水平升高。此外,免疫分型显示,肥胖动物的各种细胞,如 CD8+ 和 CD4+ T 细胞、B 细胞和巨噬细胞表面的 CD44 和 CD69 表达增加。因此,高脂/高果糖联合营养是一种更适合的西方饮食模型。然而,尽管存在这些差异,这两种模型都显示出免疫表型的变化,这些变化可能与肥胖相关癌症风险的增加有关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Obesity
医学-内分泌学与代谢
CiteScore
10.00
自引率
2.00%
发文量
221
审稿时长
3 months
期刊介绍:
The International Journal of Obesity is a multi-disciplinary forum for research describing basic, clinical and applied studies in biochemistry, physiology, genetics and nutrition, molecular, metabolic, psychological and epidemiological aspects of obesity and related disorders.
We publish a range of content types including original research articles, technical reports, reviews, correspondence and brief communications that elaborate on significant advances in the field and cover topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


