Photolytic Mass Loss of Humic Substances Measured with a Quartz Crystal Microbalance
IF 2.9
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Laboratory studies have shown that photolytic mass loss can be a significant sink for secondary organic aerosol (SOA). Here, we use a quartz crystal microbalance to measure mass loss of Suwannee River Humic Acid (SRHA) and Suwannee River Fulvic Acid (SRFA), surrogates for SOA, exposed to 254, 300, and 405 nm radiation over the course of 24 h. We find that the photolytic mass loss rates of these materials are comparable to those for laboratory-generated limonene and toluene SOA material from the study of Baboomian et al, ACS Earth Space Chem. 2020, 4, 1078. Scaling our results to ambient conditions, we estimate that humic substances in aerosols can lose as much as 8% by mass in the first day of exposure in the atmosphere, equivalent to 0.025% of JNO2, the photolysis rate of nitrogen dioxide. By using zero air instead of nitrogen, we also find that the presence of oxygen accelerates the photolytic mass loss rate by a factor of 2 to 4 at all wavelengths suggesting a potential role for reactive oxygen species. UV photolysis of an aqueous SRFA solution demonstrated both photobleaching at UV wavelengths and photoenhancement at visible wavelengths. Ultrahigh-resolution mass spectrometric analysis showed that condensed-phase SRFA photolysis led to decreased intensity in the 100–300 m/z range while aqueous SRFA photolysis resulted in an increase in intensity in the same range. This work reaffirms that photolytic mass loss is a potentially significant sink for SOA, but only on the time scale of a day or two and demonstrates that SRHA and SRFA are suitable surrogates for atmospheric SOA with respect to photolytic mass loss.
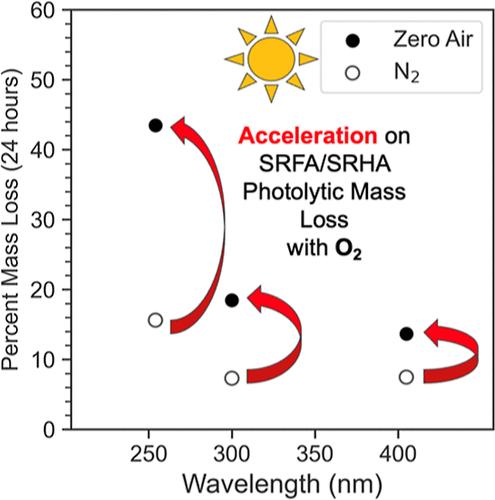
用石英晶体微天平测量腐殖质的光解质量损失
实验室研究表明,光解质量损失是二次有机气溶胶(SOA)的重要吸收汇。在此,我们使用石英晶体微天平测量了苏瓦尼河腐植酸(SRHA)和苏瓦尼河富酸(SRFA)(SOA 的替代物)在 254、300 和 405 纳米辐射下暴露 24 小时的质量损失。 我们发现,这些材料的光解质量损失率与 Baboomian 等人的研究(ACS Earth Space Chem.2020, 4, 1078.将我们的结果放大到环境条件下,我们估计气溶胶中的腐殖质在暴露于大气的第一天就会损失高达 8% 的质量,相当于二氧化氮光解率 JNO2 的 0.025%。通过使用零空气而不是氮气,我们还发现氧气的存在会使所有波长的光解质量损失率加快 2 到 4 倍,这表明活性氧可能起了作用。SRFA 水溶液的紫外光解显示了紫外波长下的光漂白和可见光波长下的光增强。超高分辨率质谱分析表明,凝聚相 SRFA 光解导致 100-300 m/z 范围内的强度降低,而水溶液 SRFA 光解则导致同一范围内的强度增加。这项工作再次证实,光解质量损失是 SOA 潜在的重要吸收汇,但只在一两天的时间范围内,并证明 SRHA 和 SRFA 是大气 SOA 光解质量损失的合适替代物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Earth and Space Chemistry
Earth and Planetary Sciences-Geochemistry and Petrology
CiteScore
5.30
自引率
11.80%
发文量
249
期刊介绍:
The scope of ACS Earth and Space Chemistry includes the application of analytical, experimental and theoretical chemistry to investigate research questions relevant to the Earth and Space. The journal encompasses the highly interdisciplinary nature of research in this area, while emphasizing chemistry and chemical research tools as the unifying theme. The journal publishes broadly in the domains of high- and low-temperature geochemistry, atmospheric chemistry, marine chemistry, planetary chemistry, astrochemistry, and analytical geochemistry. ACS Earth and Space Chemistry publishes Articles, Letters, Reviews, and Features to provide flexible formats to readily communicate all aspects of research in these fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


