Nickel-catalyzed cyclization of alkynyl nitriles with isocyanide: An expedient way to the synthesis of polysubstituted pyrroles
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We herein describe a nickel-catalyzed cascade cyclization of alkynyl nitriles with tert-butylisocyanide under simple reaction conditions. A couple of polysubstituted pyrroles were obtained in moderate yields and with excellent regioselectivity in some cases. The products from this protocol are synthetically useful since the parent pyrrole ring bears one free amino and two cyano groups which enable diverse late-stage transformations.
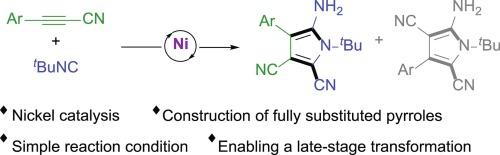
镍催化的炔腈与异氰酸酯的环化反应:合成多取代吡咯的便捷方法
我们在此介绍一种在简单反应条件下,由镍催化的炔腈与叔丁基异氰酸酯的级联环化反应。我们以中等产率获得了几种多取代的吡咯,在某些情况下具有极佳的区域选择性。由于母体吡咯环上带有一个游离氨基和两个氰基,可以进行多种后期转化,因此该方案的产物在合成方面非常有用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


