Exploring bat-inspired cyclic tryptophan diketopiperazines as ABCB1 Inhibitors
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
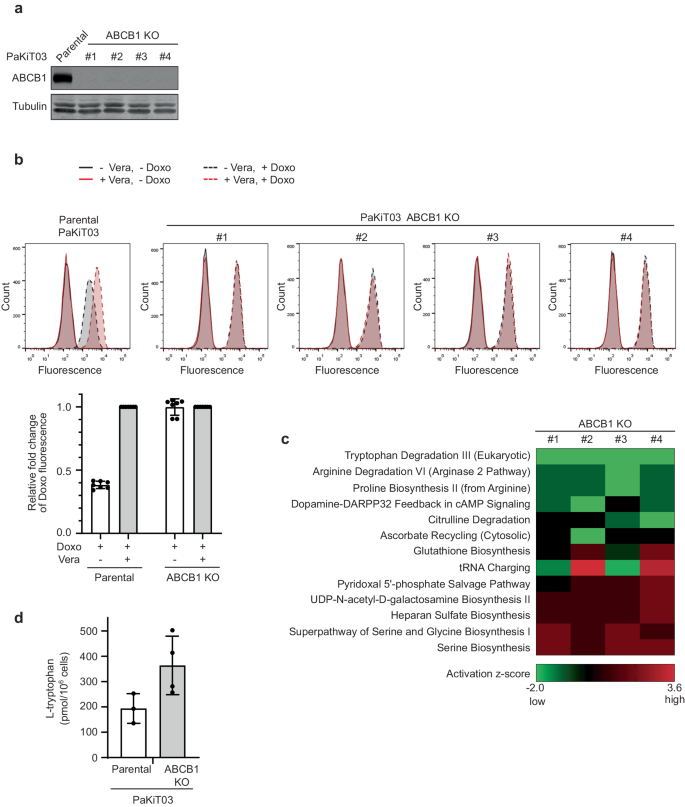
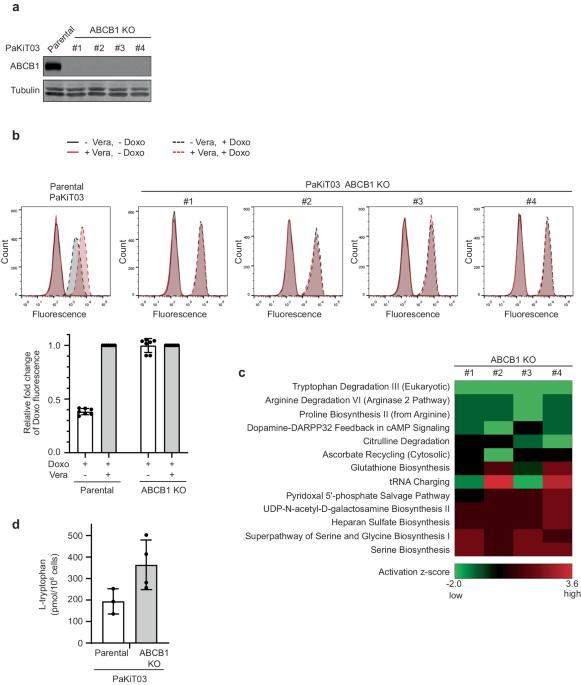
Chemotherapy-induced drug resistance remains a major cause of cancer recurrence and patient mortality. ATP binding cassette subfamily B member 1 (ABCB1) transporter overexpression in tumors contributes to resistance, yet current ABCB1 inhibitors have been unsuccessful in clinical trials. To address this challenge, we propose a new strategy using tryptophan as a lead molecule for developing ABCB1 inhibitors. Our idea stems from our studies on bat cells, as bats have low cancer incidences and high ABCB1 expression. We hypothesized that potential ABCB1 substrates in bats could act as competitive inhibitors in humans. By molecular simulations of ABCB1-substrate interactions, we generated a benzylated Cyclo-tryptophan (C3N-Dbn-Trp2) that inhibits ABCB1 activity with efficacy comparable to or better than the classical inhibitor, verapamil. C3N-Dbn-Trp2 restored chemotherapy sensitivity in drug-resistant human cancer cells with no adverse effect on cell proliferation. Our unique approach presents a promising lead toward developing effective ABCB1 inhibitors to treat drug-resistant cancers. ATP-binding cassette transporter ABCB1 is known to be involved in drug resistance in cancer treatment, however, current ABCB1 inhibitors have not been successful in clinical trials due to their potential cytotoxicity. Here, the authors hypothesize that potential ABCB1 substrates in bats could act as competitive inhibitors against ABCB1 in humans, and identify the tryptophan structure as a promising lead structure for the development of non-toxic ABCB1 inhibitors.
探索蝙蝠启发的环色氨酸二酮哌嗪作为 ABCB1 抑制剂。
化疗引起的耐药性仍然是癌症复发和患者死亡的主要原因。ATP结合盒B亚家族成员1(ABCB1)转运体在肿瘤中的过度表达是导致耐药性的原因之一,但目前的ABCB1抑制剂在临床试验中并不成功。为了应对这一挑战,我们提出了一种以色氨酸为先导分子开发 ABCB1 抑制剂的新策略。我们的想法源于对蝙蝠细胞的研究,因为蝙蝠癌症发病率低,而 ABCB1 表达量高。我们假设,蝙蝠体内潜在的 ABCB1 底物可以作为人类的竞争性抑制剂。通过对 ABCB1-底物相互作用的分子模拟,我们生成了一种苄基环色氨酸(C3N-Dbn-Trp2),它对 ABCB1 活性的抑制效果与经典抑制剂维拉帕米相当,甚至更好。C3N-Dbn-Trp2 恢复了耐药人类癌细胞对化疗的敏感性,且对细胞增殖无不良影响。我们的独特方法为开发有效的 ABCB1 抑制剂治疗耐药性癌症提供了一个前景广阔的线索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


