Structural basis of tethered agonism and G protein coupling of protease-activated receptors
IF 28.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
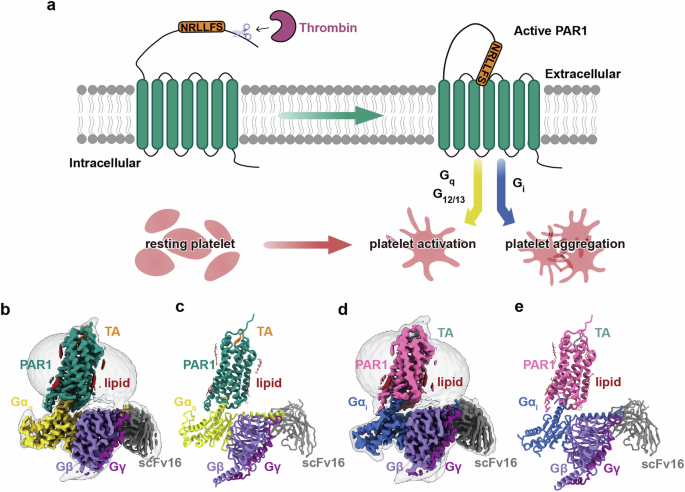
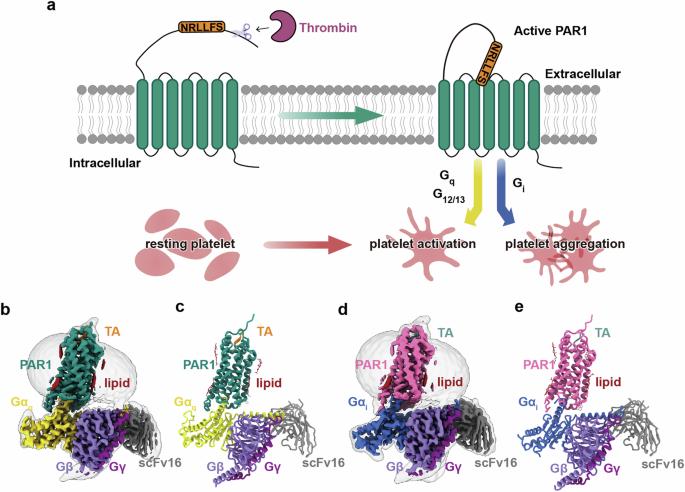
Protease-activated receptors (PARs) are a unique group within the G protein-coupled receptor superfamily, orchestrating cellular responses to extracellular proteases via enzymatic cleavage, which triggers intracellular signaling pathways. Protease-activated receptor 1 (PAR1) is a key member of this family and is recognized as a critical pharmacological target for managing thrombotic disorders. In this study, we present cryo-electron microscopy structures of PAR1 in its activated state, induced by its natural tethered agonist (TA), in complex with two distinct downstream proteins, the Gq and Gi heterotrimers, respectively. The TA peptide is positioned within a surface pocket, prompting PAR1 activation through notable conformational shifts. Contrary to the typical receptor activation that involves the outward movement of transmembrane helix 6 (TM6), PAR1 activation is characterized by the simultaneous downward shift of TM6 and TM7, coupled with the rotation of a group of aromatic residues. This results in the displacement of an intracellular anion, creating space for downstream G protein binding. Our findings delineate the TA recognition pattern and highlight a distinct role of the second extracellular loop in forming β-sheets with TA within the PAR family, a feature not observed in other TA-activated receptors. Moreover, the nuanced differences in the interactions between intracellular loops 2/3 and the Gα subunit of different G proteins are crucial for determining the specificity of G protein coupling. These insights contribute to our understanding of the ligand binding and activation mechanisms of PARs, illuminating the basis for PAR1’s versatility in G protein coupling.
蛋白酶激活受体的系链激动作用和 G 蛋白耦合的结构基础
蛋白酶激活受体(PAR)是 G 蛋白偶联受体超家族中的一个独特群体,它通过酶裂解来协调细胞对细胞外蛋白酶的反应,从而触发细胞内的信号通路。蛋白酶激活受体 1(PAR1)是这一家族的关键成员,被认为是治疗血栓性疾病的重要药物靶点。在这项研究中,我们展示了 PAR1 在其天然系链激动剂(TA)诱导下,分别与两种不同的下游蛋白(Gq 和 Gi 杂三聚体)复合后的活化状态的冷冻电镜结构。TA 肽位于表面口袋中,通过显著的构象转变促使 PAR1 激活。与涉及跨膜螺旋 6(TM6)向外移动的典型受体激活不同,PAR1 激活的特点是 TM6 和 TM7 同时向下移动,加上一组芳香族残基的旋转。这导致了细胞内阴离子的移位,为下游 G 蛋白的结合创造了空间。我们的研究结果描述了 TA 的识别模式,并强调了 PAR 家族中第二个胞外环在与 TA 形成 β 片时的独特作用,这是其他 TA 激活受体中未观察到的特征。此外,细胞内环 2/3 与不同 G 蛋白的 Gα 亚基之间相互作用的细微差别对于确定 G 蛋白耦合的特异性至关重要。这些见解有助于我们理解 PAR 的配体结合和激活机制,阐明了 PAR1 在 G 蛋白耦合中的多功能性的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


