Organocatalytic route to the enantioselective synthesis of syn/anti-α-hydrazino-γ-fluoro alcohols
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A general organocatalytic method has been developed for the asymmetric synthesis of α-hydrazino-γ-fluoro alcohols, a precursor to syn/anti-1,3-fluoro amines. The strategy employs α-fluorination catalyzed by proline-derived catalyst, (S)-α,α-bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether followed by Horner−Wadsworth−Emmons (HWE) olefination of aldehydes, and proline-catalyzed α-amination as the key steps. The title compounds showed excellent diastereoselectivity (up to 99:1) and enantioselectivity (up to 99 %).
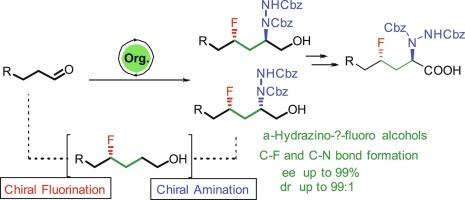
对映/反-α-肼-γ-氟醇的有机催化合成路线
本研究开发了一种通用的有机催化方法,用于不对称合成α-肼-γ-氟醇(合成/反-1,3-氟胺的前体)。该策略以脯氨酸衍生催化剂催化的α-氟化反应、(S)-α,α-双[3,5-双(三氟甲基)苯基]-2-吡咯烷甲醇三甲基硅基醚、醛的霍纳-沃兹沃斯-埃蒙斯(HWE)烯化反应以及脯氨酸催化的α-氨基反应为关键步骤。标题化合物显示出优异的非对映选择性(高达 99:1)和对映选择性(高达 99%)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


