Efficacy and Safety of Azvudine in Patients With COVID-19 in China: A Meta-Analysis of Observational Studies
Abstract
Background
Azvudine (FNC) is a novel small molecule antiviral drug for treating COVID-19 that is available only on the Chinese market. Despite being recommended for treating COVID-19 by the Chinese guidelines, its efficacy and safety are still unclear. This study aimed to evaluate the protective effect of FNC on COVID-19 outcomes and its safety.
Methods
We followed the PRISMA 2020 guidelines and searched the PubMed, Embase, Web of Science, Scopus, and China National Knowledge Infrastructure (CNKI) databases to evaluate studies on the effectiveness of FNC in treating COVID-19 in China, focusing on mortality and overall outcomes. Additionally, its impact on the length of hospital stay (LOHS), time to first nucleic acid negative conversion (T-FNANC), and adverse events was evaluated. The inclusion criterion was that the studies were published from July 2021 to April 10, 2024. This study uses the ROBINS-I tool to assess bias risk and employs the GRADE approach to evaluate the certainty of the evidence.
Results
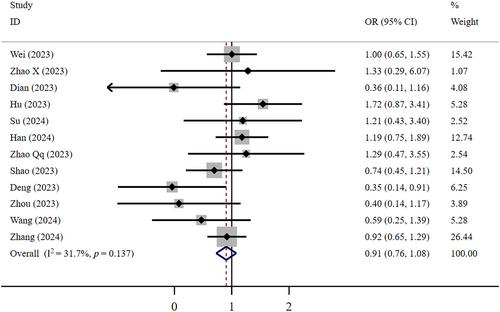
The meta-analysis included 24 retrospective studies involving a total of 11 830 patients. Low-certainty evidence revealed no significant difference in mortality (OR = 0.91, 95% CI: 0.76–1.08) or LOHS (WMD = −0.24, 95% CI: −0.83 to 0.35) between FNC and Paxlovid in COVID-19 patients. Low-certainty evidence shows that the T-FNANC was longer (WMD = 1.95, 95% CI: 0.36–3.53). Compared with the Paxlovid group, low-certainty evidence shows the FNC group exhibited a worse composite outcome (OR = 0.77, 95% CI: 0.63–0.95) and fewer adverse events (OR = 0.63, 95% CI: 0.46–0.85). Compared with supportive treatment, low certainty shows FNC significantly reduced the mortality rate in COVID-19 patients (OR = 0.61, 95% CI: 0.51–0.74) and decreased the composite outcome (OR = 0.67, 95% CI: 0.50–0.91), and very low certainty evidence shows significantly decreased the T-FNANC (WMD = −4.62, 95% CI: −8.08 to −1.15). However, in very low certainty, there was no significant difference in LOHS (WMD = −0.70, 95% CI: −3.32 to 1.91) or adverse events (OR = 1.97, 95% CI: 0.48–8.17).
Conclusions
FNC appears to be a safe and potentially effective treatment for COVID-19 in China, but further research with larger, high-quality studies is necessary to confirm these findings. Due to the certainty of the evidence and the specific context of the studies conducted in China, caution should be exercised when considering whether the results are applicable worldwide.
Trial Registration
PROSPERO number: CRD42024520565

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


