Nonsense CD247 mutations show dominant-negative features in T-cell receptor expression and function
IF 11.4
1区 医学
Q1 ALLERGY
引用次数: 0
Abstract
Background
The invariant TCR ζ/CD247 homodimer is crucial for TCR/CD3 expression and signaling through its 3 immunoreceptor tyrosine-based activation motifs (ITAMs). Homozygous null mutations in CD247 lead to immunodeficiency, while carriers exhibit 50% reduced surface CD3. It is unclear whether carriers of other CD247 variants show dominant-negative effects.
Objective
We sought to analyze and model the potential impact on T-cell receptor (TCR) expression and function of heterozygous nonsense CD247 mutations found in patients with signs of immunodeficiency or autoimmunity.
Methods
Jurkat T cells, either wild-type (WT) or CRISPR/Cas9-edited CD247-deficient (ZKO), were lentivirally transduced with WT CD247 or mutations ablating 1 (Q142X), 2 (Q101X), or 3 (Q70X) ITAMs.
Results
Three patients from unrelated families were studied. Two heterozygous nonsense CD247 mutations were identified (p.Y152X and p.Q101X), which affected ITAM-3 and ITAM-2 and ITAM-3, respectively. Both mutations were associated with low surface CD3 expression and normal intracellular CD247 levels using a transmembrane-specific antibody, but very low intracellular CD247 levels using an ITAM-3–specific one, suggesting the presence of truncated variants in T cells. Transduction of the mutations lacking 1, 2, or 3 ITAMs into ZKO cells could not restore normal surface CD3 expression (only 60%, 22%, and 10%, respectively), whereas in WT cells, normal surface CD3 expression was reduced (to 39%, 19%, and 9% of normal levels), and both effects were dependent on ITAM number. All 6 transfectants showed reduced CD69 induction (25% to 50%), indicating that they were unable to signal downstream properly, neither isolated nor associated with WT CD247.
Conclusions
Our results suggest that CD247 variants lacking ITAMs due to nonsense, but not null, mutations are defective for normal TCR assembly and exert a dominant-negative effect on TCR expression and signaling in vitro. This, in turn, may correlate with clinical features in vivo.
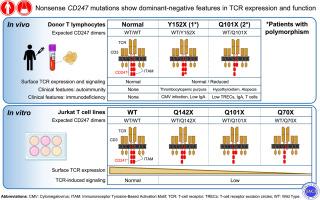
有义 CD247 突变在 TCR 表达和功能方面表现出显性阴性特征。
背景:不变TCRζ/CD247同源二聚体通过其三个基于免疫受体酪氨酸的激活基序(ITAM)对TCR/CD3的表达和信号转导至关重要。CD247 同源无效突变会导致免疫缺陷,而携带者的表面 CD3 会减少 50%。目前还不清楚其他 CD247 变异的携带者是否会出现显性阴性效应:目的:分析和模拟在有免疫缺陷或自身免疫迹象的患者中发现的杂合子无义 CD247 突变对 TCR 表达和功能的潜在影响:方法:用野生型 CD247 或消减一个(Q142X)、两个(Q101X)或三个(Q70X)ITAM 的突变慢病毒转染野生型(WT)或 CRISPR/Cas9 编辑的 CD247 缺失型(ZKO)的 Jurkat T 细胞:研究了来自非亲缘家庭的三名患者。研究发现了两个杂合子CD247无义突变(p.Y152X和p.Q101X),分别影响ITAM-3和ITAM-2+3。这两种突变都与低表面CD3表达、正常细胞内CD247水平(使用跨膜特异性抗体)以及极低细胞内CD247水平(使用ITAM-3特异性抗体)有关,这表明T细胞中存在截短变体。将缺少 1、2 或 3 个 ITAM 的突变体转入 ZKO 无法恢复正常的表面 CD3 表达(分别只有 60%、22% 和 10%),而转入 WT 则会降低其表达(分别降至正常水平的 39%、19% 和 9%),这两种效应都与 ITAM 数量有关。所有六种转染体都显示出 CD69 诱导减少(25%-50%),这表明它们既不能与野生型 CD247 分离,也不能与野生型 CD247 相关联,从而无法向下游适当地发出信号:我们的研究结果表明,由于无义突变(而非无效突变)而缺乏 ITAMs 的 CD247 变体在正常 TCR 组装方面存在缺陷,并在体外对 TCR 表达和信号转导产生显性负效应。这反过来又可能与体内的临床特征相关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
25.90
自引率
7.70%
发文量
1302
审稿时长
38 days
期刊介绍:
The Journal of Allergy and Clinical Immunology is a prestigious publication that features groundbreaking research in the fields of Allergy, Asthma, and Immunology. This influential journal publishes high-impact research papers that explore various topics, including asthma, food allergy, allergic rhinitis, atopic dermatitis, primary immune deficiencies, occupational and environmental allergy, and other allergic and immunologic diseases. The articles not only report on clinical trials and mechanistic studies but also provide insights into novel therapies, underlying mechanisms, and important discoveries that contribute to our understanding of these diseases. By sharing this valuable information, the journal aims to enhance the diagnosis and management of patients in the future.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


