Tau-mediated synaptic dysfunction is coupled with HCN channelopathy
Abstract
INTRODUCTION
In tauopathies, altered tau processing correlates with impairments in synaptic density and function. Changes in hyperpolarization-activated cyclic nucleotide-gated (HCN) channels contribute to disease-associated abnormalities in multiple neurodegenerative diseases.
METHODS
To investigate the link between tau and HCN channels, we performed histological, biochemical, ultrastructural, and functional analyses of hippocampal tissues from Alzheimer's disease (AD), age-matched controls, Tau35 mice, and/or Tau35 primary hippocampal neurons.
RESULTS
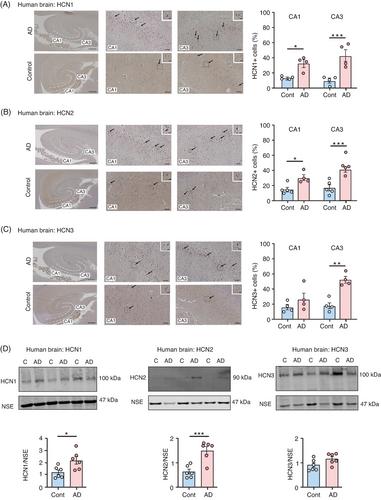
Expression of specific HCN channels is elevated in post mortem AD hippocampus. Tau35 mice develop progressive abnormalities including increased phosphorylated tau, enhanced HCN channel expression, decreased dendritic branching, reduced synapse density, and vesicle clustering defects. Tau35 primary neurons show increased HCN channel expression enhanced hyperpolarization-induced membrane voltage “sag” and changes in the frequency and kinetics of spontaneous excitatory postsynaptic currents.
DISCUSSION
Our findings are consistent with a model in which pathological changes in tauopathies impact HCN channels to drive network-wide structural and functional synaptic deficits.
Highlights
- Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels are functionally linked to the development of tauopathy.
- Expression of specific HCN channels is elevated in the hippocampus in Alzheimer's disease and the Tau35 mouse model of tauopathy.
- Increased expression of HCN channels in Tau35 mice is accompanied by hyperpolarization-induced membrane voltage “sag” demonstrating a detrimental effect of tau abnormalities on HCN channel function.
- Tau35 expression alters synaptic organization, causing a loosened vesicle clustering phenotype in Tau35 mice.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


