Remote site-selective arene C–H functionalization enabled by N-heterocyclic carbene organocatalysis
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
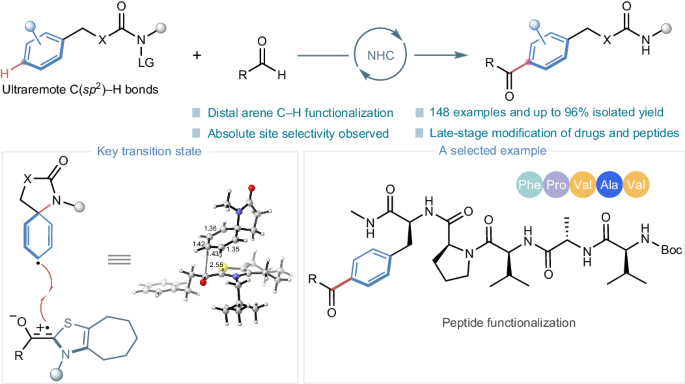
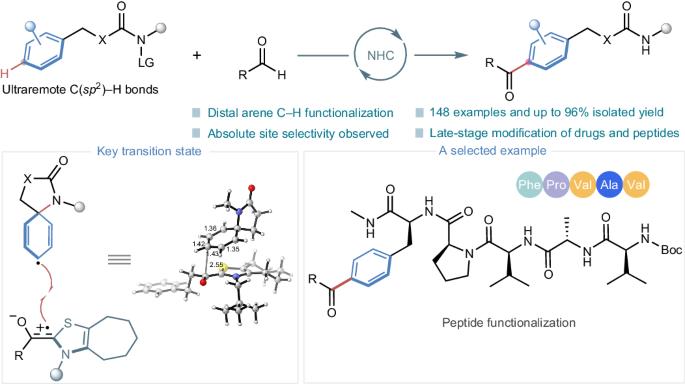
Catalytic site-selective functionalization of distal C–H bonds represents a formidable challenge in organic synthesis. Particularly, the precise functionalization of distal aromatic C(sp2)–H bonds remains largely unexplored. Here we present a highly para-selective acylation strategy to target ultraremote aryl C(sp2)–H bonds, eight chemical bonds away from an activated functionality, through radical N-heterocyclic carbene organocatalysis. This method is developed on the basis of a unique single-electron pathway involving the site-selective activation of aryl C–H bonds by a nitrogen-centred radical generated in situ. Importantly, this organocatalytic approach shows potential for the functionalization of drugs, amino acids and peptides, thus highlighting its importance for medicinal chemistry. Our investigation encompassed meticulous mechanistic studies, including control experiments and density functional theory calculations, to unravel the intricacies behind the observed site selectivity and shed light on the mechanism of radical N-heterocyclic carbene organocatalysis. The precise functionalization of distant aromatic C(sp2)–H bonds remains largely unexplored. Here the authors report a para-selective acylation strategy to target remote aryl C(sp2)–H bonds away from an activated functionality through radical N-heterocyclic carbene organocatalysis.
通过 N-杂环碳烯有机催化实现远程位点选择性炔烃 C-H 功能化
远端 C-H 键的催化位点选择性官能化是有机合成中的一项艰巨挑战。尤其是远端芳香族 C(sp2)-H 键的精确官能化在很大程度上仍未得到探索。在此,我们提出了一种高准选择性酰化策略,通过自由基 N- 异环碳烯有机催化,以距离活化官能团八个化学键的超远程芳基 C(sp2)-H 键为目标。这种方法是在一种独特的单电子途径基础上开发出来的,该途径涉及以氮为中心的自由基在原位生成的芳基 C-H 键的位点选择性活化。重要的是,这种有机催化方法显示了药物、氨基酸和肽功能化的潜力,从而凸显了其在药物化学中的重要性。我们的研究涵盖了细致的机理研究,包括对照实验和密度泛函理论计算,以揭示观察到的位点选择性背后的复杂性,并阐明自由基 N-杂环碳烯有机催化的机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


