Electrocatalytic cyclic deracemization enabled by a chemically modified electrode
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Redox chemistry, which is frequently encountered in the formation of new bonds and stereocentres, relies on the compatibility of redox potentials. Despite recent advances, achieving a general electrocatalytic cyclic deracemization process without stoichiometric redox reagents remains a formidable challenge. Here we show that electrocatalytic cyclic deracemization of secondary alcohols can be accomplished through sequential iridium-catalysed enantioselective anodic dehydrogenation and rhodium-catalysed cathodic hydrogenation, utilizing metal hydride catalysis. A considerable hurdle arises as stronger hydride donors necessitate parent metal complexes to possess low reduction potentials, resulting in inherent redox potential incompatibility. Nonetheless, we overcame this incompatibility by leveraging a recyclable rhodium-catalyst-modified electrode as the cathode—an accomplishment that homogeneous rhodium catalysis could not achieve. Our approach enables chemoselective stereochemical editing of bioactive compounds with remarkable functional group tolerance. Surface characterization and mechanistic studies showcased the unique advantages conferred by the chemically modified electrode. Methods for electrocatalytic deracemization remained elusive. Now, electrocatalytic cyclic deracemization of alcohols is reported, involving sequential anodic dehydrogenation and cathodic hydrogenation using two distinct metal hydride catalysts and a chemically modified electrode to avoid redox incompatibility.
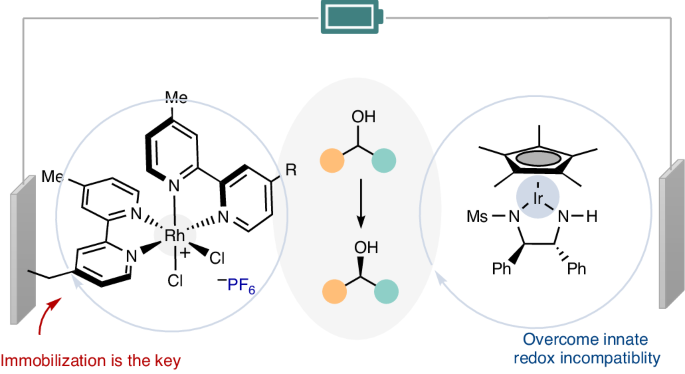
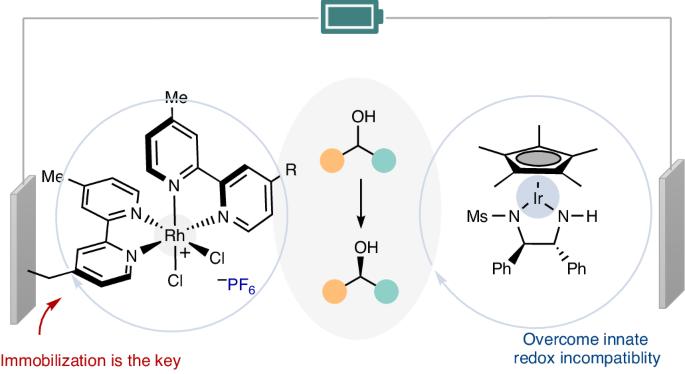
通过化学修饰电极实现电催化循环脱盐
氧化还原化学在新键和立体中心的形成过程中经常遇到,它依赖于氧化还原电位的兼容性。尽管最近取得了一些进展,但要在不使用化学计量氧化还原试剂的情况下实现一般的电催化环状去甲基化过程,仍然是一项艰巨的挑战。在这里,我们展示了利用金属氢化物催化,通过铱催化的对映体选择性阳极脱氢和铑催化的阴极氢化顺序,可以实现仲醇的电催化循环脱氢。由于较强的氢化物供体要求母体金属复合物具有较低的还原电位,从而导致固有的氧化还原电位不相容,因此出现了相当大的障碍。不过,我们利用可回收的铑催化剂修饰电极作为阴极,克服了这种不兼容性--这是同质铑催化无法实现的。我们的方法实现了生物活性化合物的化学选择性立体化学编辑,并具有显著的官能团耐受性。表面表征和机理研究展示了化学修饰电极的独特优势。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


