Adoptive transfer of personalized neoantigen-reactive TCR-transduced T cells in metastatic colorectal cancer: phase 2 trial interim results
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
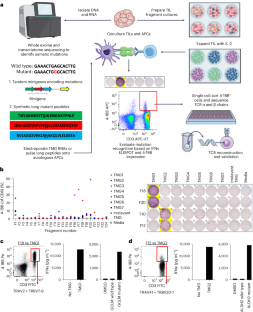
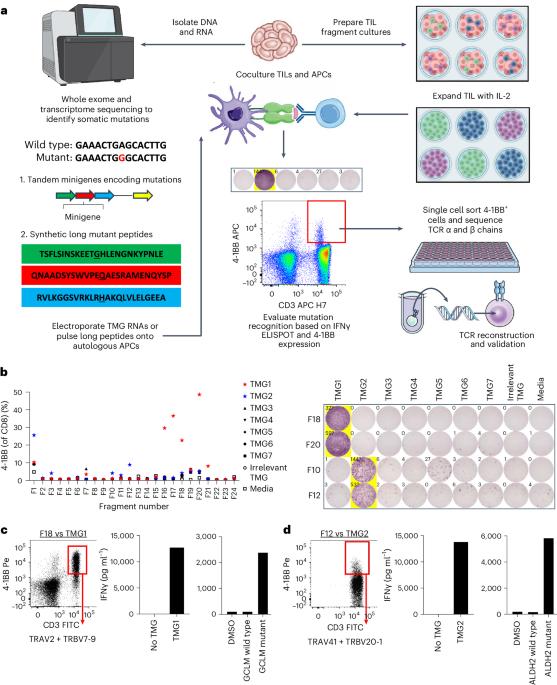
Adoptive cell transfer (ACT) with neoantigen-reactive T lymphocytes can mediate cancer regression. Here we isolated unique, personalized, neoantigen-reactive T cell receptors (TCRs) from tumor-infiltrating lymphocytes of patients with metastatic gastrointestinal cancers and incorporated the TCR α and β chains into gamma retroviral vectors. We transduced autologous peripheral blood lymphocytes and adoptively transferred these cells into patients after lymphodepleting chemotherapy. In a phase 2 single-arm study, we treated seven patients with metastatic, mismatch repair-proficient colorectal cancers who had progressive disease following multiple previous therapies. The primary end point of the study was the objective response rate as measured using RECIST 1.1, and the secondary end points were safety and tolerability. There was no prespecified interim analysis defined in this study. Three patients had objective clinical responses by RECIST criteria including regressions of metastases to the liver, lungs and lymph nodes lasting 4 to 7 months. All patients received T cell populations containing ≥50% TCR-transduced cells, and all T cell populations were polyfunctional in that they secreted IFNγ, GM-CSF, IL-2 and granzyme B specifically in response to mutant peptides compared with wild-type counterparts. TCR-transduced cells were detected in the peripheral blood of five patients, including the three responders, at levels ≥10% of CD3+ cells 1 month post-ACT. In one patient who responded to therapy, ~20% of CD3+ peripheral blood lymphocytes expressed transduced TCRs more than 2 years after treatment. This study provides early results suggesting that ACT with T cells genetically modified to express personalized neoantigen-reactive TCRs can be tolerated and can mediate tumor regression in patients with metastatic colorectal cancers. ClinicalTrials.gov registration: NCT03412877 . Preliminary findings in seven patients with mismatch repair-proficient metastatic colorectal cancer who were treated in the context of a phase 2 trial show that adoptive transfer of autologous peripheral blood T lymphocytes that were retrovirally transduced with personalized neoantigen-reactive T cell receptors can be safe and induce early clinical responses.
个性化新抗原反应TCR转导T细胞在转移性结直肠癌中的适应性转移:2期试验中期结果
新抗原反应T淋巴细胞的适应性细胞转移(ACT)可促进癌症消退。在这里,我们从转移性胃肠癌患者的肿瘤浸润淋巴细胞中分离出了独特的个性化新抗原反应T细胞受体(TCR),并将TCR α和β链整合到γ逆转录病毒载体中。我们转导了自体外周血淋巴细胞,并将这些细胞收养性转移给接受淋巴清除化疗的患者。在一项 2 期单臂研究中,我们治疗了七名错配修复功能良好的转移性结直肠癌患者,这些患者在接受了之前的多种疗法后病情有所进展。研究的主要终点是使用 RECIST 1.1 测量的客观反应率,次要终点是安全性和耐受性。这项研究没有进行预先指定的中期分析。根据 RECIST 标准,3 名患者出现了客观临床反应,包括肝脏、肺部和淋巴结转移的消退,持续时间为 4 至 7 个月。所有患者接受的T细胞群中都含有≥50%的TCR转导细胞,而且所有T细胞群都具有多功能性,与野生型T细胞群相比,它们在应答突变肽时会分泌特异性IFNγ、GM-CSF、IL-2和颗粒酶B。在包括三名应答者在内的五名患者的外周血中检测到了 TCR 转导的细胞,其水平≥CD3+细胞的 10%。一名对治疗有反应的患者在治疗 2 年多后,约 20% 的 CD3+ 外周血淋巴细胞表达了转导的 TCR。这项研究提供的早期结果表明,转基因表达个性化新抗原反应TCR的T细胞ACT可以被转移性结直肠癌患者耐受,并能促进肿瘤消退。ClinicalTrials.gov 注册:NCT03412877。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


