House dust mite immunotherapy: A real-world, prescription data-based analysis
Abstract
Background
House dust mite (HDM) sensitisation can contribute to the development of allergic rhinoconjunctivitis (AR) or allergic asthma (AA). As treatment, allergen immunotherapy (AIT) is a promising approach, since it aims building immunotolerance against allergens, therewith establishing long-term efficacy. The evaluation of AIT has been investigated in many randomised controlled trials, whereas few real-world evidence studies are available.
Methods
We used data from the longitudinal prescription data base IQVIA™ LRx. Data on initial AIT prescriptions against HDM from January 2009 to December 2013 was analysed regarding treatment (subcutaneous AIT with either depigmented polymerised allergen extract [dSCIT] or other allergens [oSCIT], or sublingual immunotherapy [SLIT]) and treatment duration. Treatment groups were compared with a control group of AR patients not receiving AIT. Data on symptomatic medication was collected until February 2017 and progression of AR and AA was compared.
Results
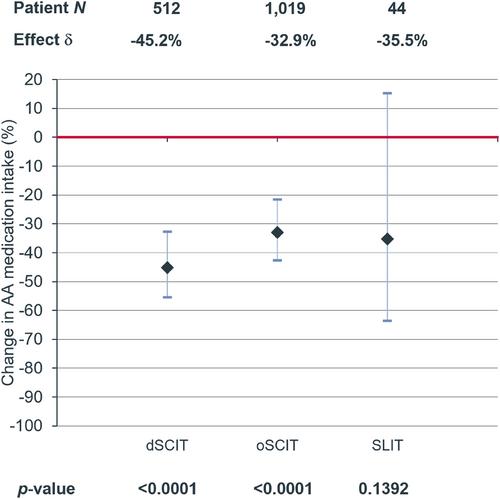
Data of 7260 patients with AIT prescriptions and of 21,780 control patients was analysed. AIT was associated with a significant decrease of AR medication intake compared with control (dSCIT: −34.0%, p < 0.0001; oSCIT: −25.7%, p < 0.0001; SLIT: −37.7%, p = 0.0026). In asthmatics, SCIT was associated with a significant decrease of asthma medication compared with control (dSCIT: −45.2%, p < 0.0001; oSCIT: −32.9%, p < 0.0001). Further, a significantly reduced likelihood for onset of asthma medication was demonstrated in patients treated with SCIT compared with controls (dSCIT OR: 0.759, p = 0.0476; oSCIT OR: 0.815, p = 0.0339).
Conclusion
Real-world data analyses indicate that AIT, particularly given via a subcutaneous route, reduces the need of medication against AR and AA and might delay the onset of asthma medication in patients with AR.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


