A combined experimental and theoretical study on the mechanism of catalytic oxidation of lignite to carboxylic acids by iron
Abstract
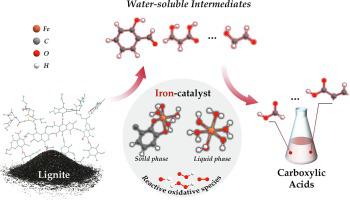
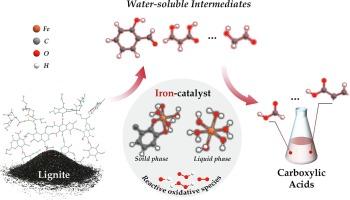
The oxygen-oxidation of lignite to carboxylic acids (CAs) using environmentally friendly iron catalyst represents an efficient and clean technology for lignite utilization. However, the mechanism remains unclear due to the difficulty in observing the cleavage of chemical bonds and the challenge in capturing transient intermediates and radicals. In this work, the microscopic reaction mechanism of iron-catalytic oxidation of lignite was investigated by combining experimental and theoretical methods. Catalytic oxidation experiments revealed that water-soluble intermediates generated by solid-state lignite decomposition underwent a continuous carbon–carbon bond cleavage oxidation (including oxidative cleavage of aliphatic moieties and oxidative ring-opening of aromatic moieties) to form CAs with aldehyde moieties as the direct precursor. Based on molecular dynamics simulations and density functional theory, a reaction network from lignite to CAs was proposed. The iron catalyst was present in the form of iron-aqua complexes in the liquid phase and iron-phenyl complexes in the solid phase. These iron-aqua/iron-phenyl complexes could reduce the energies of oxidation reactions, promoting the cleavage of bridge bonds, the formation of o-quinones and the oxidation of aromatic moieties, thus accelerating elementary reactions for the formation of CAs. These findings provide a theoretical support for the oxidation of lignite and an in-depth insight into iron-catalytic mechanism.

| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


