Insight into the role of a trans-AT polyketide synthase in the biosynthesis of lankacidin-type natural products
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
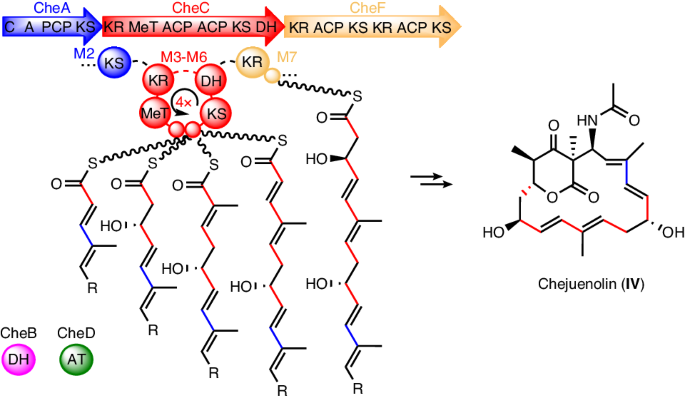
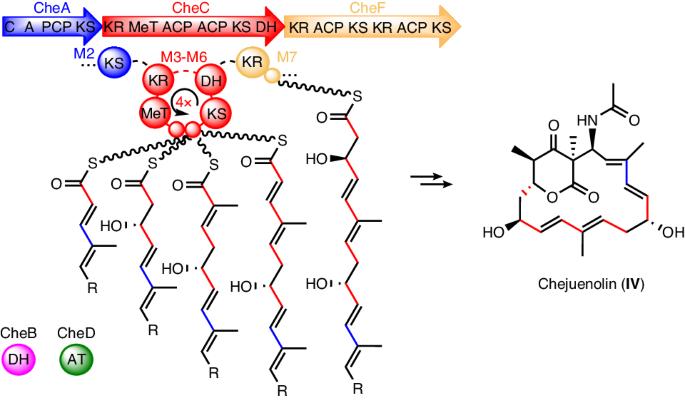
Modular polyketide synthases (type I PKSs) are biosynthetic assembly lines for synthesizing a diverse array of natural products. While most PKSs exhibit a linear module architecture, the PKS system for lankacidin-type natural products contains only five modules but carries out eight rounds of polyketide extension, challenging the collinearity rule. Here we show the distinct domain architecture of the polyketide synthase enzyme, CheC, which is central to chejuenolide biosynthesis. CheC not only dissociates from and interacts with both the preceding and succeeding PKS enzymes, creating two linear modules, but also independently assembles an unconventional module, facilitating multiple rounds of polyketide extension in the biosynthetic process. We also unveiled missing functions of certain redundant and absent domains within PKSs, fully elucidating the polyketide assembly process for lankacidin-like natural products. These findings not only reveal the biosynthetic pathway for lankacidin- and chejuenolin-type natural products but also enrich the diverse functions of PKSs, setting the stage for future rational design of PKSs. In vitro biosynthetic analysis reveals that a single trans-AT polyketide synthase (PKS) module iteratively enables five rounds of polyketide elongation and interacts with both the preceding and succeeding PKS enzymes to produce the 17-membered macrocyclic polyketide chejunolide.
透视反式 AT 多酮合成酶在兰卡苷类天然产物生物合成中的作用
模块化多酮合成酶(I 型 PKS)是合成各种天然产物的生物合成装配线。虽然大多数 PKSs 都表现出线性模块结构,但用于生产兰卡苷类天然产物的 PKS 系统仅包含五个模块,却能进行八轮聚酮延伸,这对共线性规则提出了挑战。在这里,我们展示了多酮苷合成酶 CheC 的独特结构域,它是螯合烯醇内酯生物合成的核心。CheC 不仅能与前一个和后一个 PKS 酶解离并相互作用,形成两个线性模块,还能独立组装一个非常规模块,促进生物合成过程中多轮聚酮内酯的延伸。我们还揭示了 PKS 内部某些冗余和缺失结构域的缺失功能,从而全面阐明了类兰卡苷天然产物的聚酮组装过程。这些发现不仅揭示了兰卡苷和嚼草素类天然产物的生物合成途径,而且丰富了PKSs的多种功能,为今后合理设计PKSs奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


