Iridium-catalysed synthesis of C,N,N-cyclic azomethine imines enables entry to unexplored nitrogen-rich 3D chemical space
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
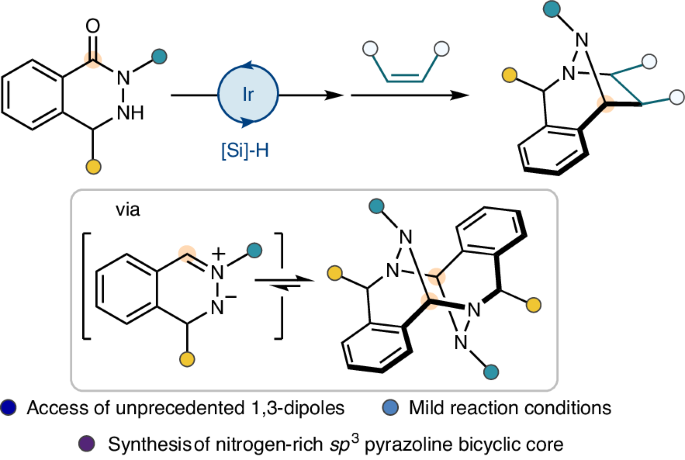
Three-dimensional nitrogen-rich bridged ring systems are of great interest in drug discovery owing to their distinctive physicochemical and structural properties. However, synthetic approaches towards N–N-bond-containing bridged heterocycles are often inefficient and require tedious synthetic strategies. Here we delineate an iridium-catalysed reductive approach to such architectures from C,N,N-cyclic hydrazide substrates using IrCl(CO)[P(OPh)3]2 and 1,1,3,3-tetramethyldisiloxane (TMDS), which provided efficient access to the unstabilized and highly reactive C,N,N-cyclic azomethine imine dipoles. These species were stable and isolable in their dimeric form, but, upon dissociation in solution, reacted with a broad range of dipolarophiles in [3 + 2] cycloaddition reactions with high yields and good diastereoselectivities, enabling the direct synthesis of nitrogen-rich sp3-hybridized pyrazoline polycyclic ring systems. Density functional theory calculations were performed to elucidate the origin of the diastereoselectivity of the cycloaddition reaction, and principal moment of inertia (PMI) analysis was conducted to enable visualization of the topological information of the dipolar cycloadducts. Three-dimensional nitrogen-rich bridged systems are of great importance in drug design. Now, a synthetic strategy enabling their preparation from readily available starting materials has been developed. This approach provides access to unstabilized C,N,N-cyclic azomethine imines, which are obtained as bench-stable dimers and undergo [3 + 2] cycloaddition reactions with various dipolarophiles.
铱催化合成 C,N,N-环氮甲基亚胺,进入未探索的富氮三维化学空间
三维富氮桥环系统具有独特的物理化学和结构特性,因此在药物发现方面具有重大意义。然而,含 N-N 键桥接杂环的合成方法往往效率低下,而且需要繁琐的合成策略。在这里,我们利用 IrCl(CO)[P(OPh)3]2 和 1,1,3,3-四甲基二硅氧烷 (TMDS),描述了一种从 C,N,N-环酰肼底物到此类结构的铱催化还原方法,它提供了高效获取未稳定和高活性 C,N,N-环氮甲基亚胺二极的途径。这些物种以二聚体形式稳定且可分离,但在溶液中解离后,会在 [3 + 2] 环加成反应中与多种偶极亲和剂发生反应,并具有高产率和良好的非对映选择性,从而能够直接合成富氮 sp3 杂化吡唑啉多环系统。研究人员进行了密度泛函理论计算,以阐明环加成反应非对映选择性的来源,并进行了主惯性矩(PMI)分析,以实现双极性环加成物拓扑信息的可视化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


