Synthesis of azahexabenzocoronenium salts through a formal [3 + 3] cycloaddition strategy
IF 20
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Heteroatom-embedded hexa-peri-hexabenzocoronene (HBC) molecules exhibit interesting properties depending on the number and position of the introduced heteroatoms and are promising materials for applications in organic electronics and supramolecular chemistry. However, their synthesis is quite limited because of the difficulty in selectively introducing heteroatoms into the HBC core, which poses a challenge in organic synthesis. Here we report a strategy for the in-solution synthesis of 3a2-azahexa-peri-hexabenzocoronenium salts, which are cationic nitrogen-embedded HBC derivatives. The synthesis was enabled by the formal [3 + 3] cycloaddition of polycyclic aromatic azomethine ylides with cyclopropenes, as a three-atom dipolarophile, followed by mechanochemical intramolecular cyclization. Furthermore, on-surface polymerization of aza-HBC precursors was performed to synthesize aza-HBC-based chevron-like graphene nanoribbons. This study provides the possibility for the further use of nitrogen-embedded HBC derivatives in a variety of potential applications. Heteroatom-embedded hexa-peri-hexabenzocoronenes (HBCs) display notable properties, but the selective incorporation of heteroatoms into the HBC core makes their synthesis difficult. Now a two-step process for the synthesis of 3a2-azahexabenzocoronenium salts is reported. The process comprises a formal [3 + 3] cycloaddition between azomethine ylides and cyclopropenes, followed by mechanochemical cyclization.
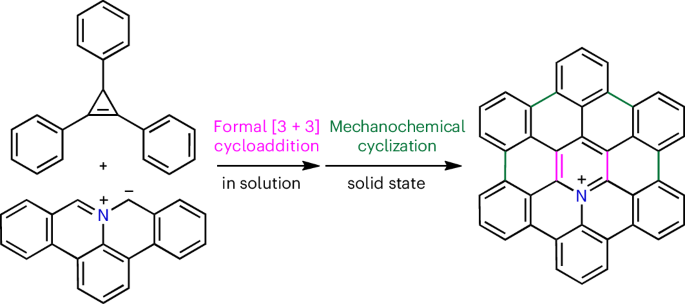
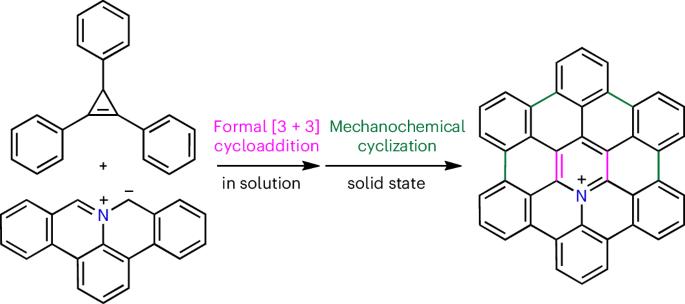
通过正规[3 + 3]环加成策略合成杂六苯并硼酸盐
根据引入杂原子的数量和位置,嵌入杂原子的六-过-六苯并二硼烯(HBC)分子会表现出有趣的特性,是有机电子学和超分子化学领域很有应用前景的材料。然而,由于难以选择性地将杂原子引入 HBC 核心,它们的合成受到很大限制,这给有机合成带来了挑战。在此,我们报告了一种在溶液中合成 3a2-azahexa-peri-hexabenzocoronenium 盐的策略,这种盐是阳离子氮嵌入 HBC 衍生物。该合成是通过多环芳香偶氮甲基酰化物与环丙烯的正式[3 + 3]环加成反应(作为三原子双极性亲和剂),然后通过机械化学分子内环化实现的。此外,还对氮杂环丁烷前体进行了表面聚合,合成了基于氮杂环丁烷的雪佛龙状石墨烯纳米带。这项研究为进一步将嵌氮六溴环十二烷衍生物用于各种潜在应用提供了可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


