Electrosynthesis of urea by using Fe2O3 nanoparticles encapsulated in a conductive metal–organic framework
IF 20
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The synthesis of urea by the electrochemical co-reduction of CO2 and nitrate is a crucial and challenging task. Catalysts typically suffer from either low Faradaic efficiency (FE) or inadequate current density, leading to a restricted yield rate of urea. Here we report ultrasmall γ-Fe2O3 nanoparticles (<2 nm) encapsulated in the pores of a conductive (40 S cm−1) metal–organic framework Ni-HITP (HITP = 2,3,6,7,10,11-hexaaminotriphenylene), resulting in a composite material, γ-Fe2O3@Ni-HITP. Under neutral conditions, γ-Fe2O3@Ni-HITP exhibited a state-of-art electrocatalytic performance for urea synthesis through the co-reduction of CO2 and nitrate in CO2-saturated 1 M KHCO3 and 0.1 M KNO3 aqueous solutions, achieving a FEurea of 67.2(6)%, a current density of −90 mA cm−2 and an high yield rate of $$20.4(2)\,{\mathrm{g}}\,{\mathrm{h}}^{-1}\,{\mathrm{g}}_{\mathrm{cat}}^{-1}$$ (7.7(1) mg h−1 cm−2), which is about five times higher than the rates of previously reported catalysts. No degradation was observed over 150 h of continuous operation at such a high yield rate. Enlarging the electrode area by 125 times yielded about 1.05(4) g of high-purity urea over 8 h. A mechanistic study revealed that Fe(III) ions in the γ-Fe2O3 nanoparticles exhibit high activity, generating the key intermediates *NH2 and *COOH. Furthermore, pairs of adjacent Fe(III) ions in the γ-Fe2O3 nanoparticles can act as highly active catalytic sites for catalysing the C–N coupling between *NH2 and *COOH, resulting in the formation of the subsequent key intermediate *CONH2, thereby contributing to the exceptionally high performance of γ-Fe2O3@Ni-HITP for urea production. Small γ-Fe2O3 nanoparticles (<2 nm) encapsulated in the pores of a conductive metal–organic framework enable the efficient electrosynthesis of urea through the co-reduction of CO2 and nitrate under neutral conditions.
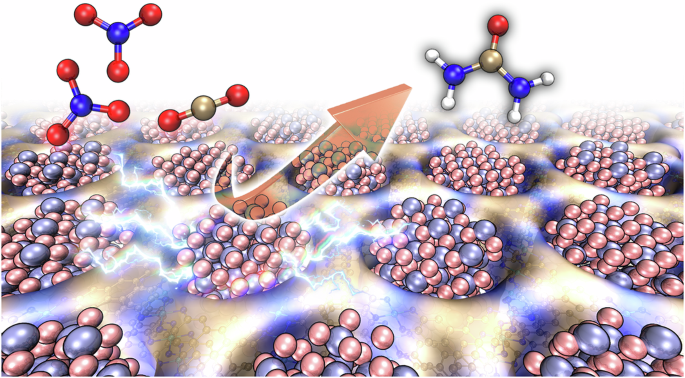
利用封装在导电金属有机框架中的 Fe2O3 纳米粒子电合成尿素
通过二氧化碳和硝酸盐的电化学共还原合成尿素是一项关键而又具有挑战性的任务。催化剂通常存在法拉第效率(FE)低或电流密度不足的问题,导致尿素的产率受到限制。在此,我们报告了超小γ-Fe2O3 纳米粒子(2 nm)被包裹在导电(40 S cm-1)金属有机框架 Ni-HITP (HITP = 2,3,6,7,10,11-六氨基三亚苯)的孔隙中,从而形成了一种复合材料--γ-Fe2O3@Ni-HITP。在中性条件下,γ-Fe2O3@Ni-HITP 在二氧化碳饱和的 1 M KHCO3 和 0.1 M KNO3 水溶液中通过 CO2 和硝酸盐的共还原作用合成尿素,表现出一流的电催化性能,FEurea 达到 67.2(6)%,电流密度为 -90 mA cm-2,产率高达 \(20.4(2)\,{\mathrm{g}}\,{\mathrm{h}}^{-1}\,{\mathrm{g}}_{\mathrm{cat}}^{-1}\) (7.7(1) mg h-1 cm-2),是之前报道的催化剂产率的五倍。在如此高的产率下,连续运行 150 小时也没有观察到降解现象。机理研究表明,γ-Fe2O3 纳米颗粒中的铁(III)离子具有很高的活性,能生成关键的中间产物 *NH2 和 *COOH。此外,γ-Fe2O3 纳米粒子中相邻的成对 Fe(III)离子可作为高活性催化位点,催化 *NH2 和 *COOH 之间的 C-N 偶联,从而形成随后的关键中间体 *CONH2,从而使 γ-Fe2O3@Ni-HITP 在尿素生产中具有极高的性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
文献相关原料
公司名称
产品信息
阿拉丁
potassium nitrate-15N
阿拉丁
potassium nitrite
阿拉丁
acetic acid
阿拉丁
N-(1-naphthyl)ethylenediamine dihydrochloride
阿拉丁
sulfanilic acid
阿拉丁
ammonium chloride (NH4Cl)
阿拉丁
sodium nitroferricyanide
阿拉丁
sodium hypochlorite (NaClO)
阿拉丁
sodium citrate
阿拉丁
sodium acetate (NaOAc)
阿拉丁
N,N-dimethylaniline (DMA)
阿拉丁
N,N-dimethylformamide (DMF)
阿拉丁
Nickel(II) acetate tetrahydrate
阿拉丁
salicylic acid
阿拉丁
sodium hydroxide (NaOH)
阿拉丁
dimethyl sulfoxide (DMSO)
阿拉丁
urea
阿拉丁
thiosemicarbazide
阿拉丁
diacetylmonoxime
阿拉丁
sulfuric acid (H2SO4)
阿拉丁
phosphoric acid
阿拉丁
potassium nitrate (KNO3)
阿拉丁
potassium bicarbonate (KHCO3)
阿拉丁
Nafion
阿拉丁
methanol
阿拉丁
iron(III) nitrate

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


