Gut Microbiota Regulate Saturated Free Fatty Acid Metabolism in Heart Failure
IF 8.3
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
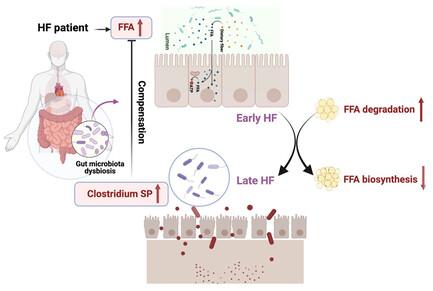
Aims: Heart failure (HF) is associated with profound changes in cardiac metabolism. At present, there is still a lack of relevant research to explore the key microbiome and their metabolites affecting the progression of HF. Herein, the interaction of gut microbiota and circulating free fatty acid (FFA) in HF patients and mice is investigated. Methods and Results: In HF patients, by applying metagenomics analysis and targeted FFA metabolomics, enriched abundance of Clostridium sporogenes (C.sp) in early and late stage of HF patients, which negatively correlated to saturated free fatty acid (SFA) levels, is identified. KEGG analysis further indicates microbiota gene enrichment in FFA degradation in early HF, and decreased gene expression in FFA synthesis in late HF. In HF mice (C57BL/6J) induced by isoproterenol (ISO), impaired intestinal permeability is observed, and decreased fecal C.sp and increased SFA are further validated. At last, by supplementing C.sp to ISO-induced HF mice, the cardiac function, fibrosis, and myocardial size are partially rescued, together with decreased circulating SFA levels. Conclusions: Clostridium abundance is increased in HF, compensating cardiac function deterioration via downregulation of circulating SFA levels. The results demonstrate that the gut microbiota–SFA axis plays an important role in HF protection, which may provide a strategic advantage for the probiotic therapy development in HF.
肠道微生物群调节心力衰竭患者的饱和游离脂肪酸代谢
目的:心力衰竭(HF)与心脏代谢的深刻变化有关。目前,仍缺乏相关研究来探讨影响心力衰竭进展的关键微生物群及其代谢产物。本文研究了高血脂患者和小鼠肠道微生物群与循环游离脂肪酸(FFA)的相互作用。方法和结果:通过应用元基因组学分析和靶向游离脂肪酸代谢组学,发现梭状芽孢杆菌(C.sp)在高血脂患者早期和晚期的丰度与饱和游离脂肪酸(SFA)水平呈负相关。KEGG 分析进一步表明,在高血脂早期,微生物群中富含脂肪酸降解的基因,而在高血脂晚期,富含脂肪酸合成的基因表达减少。在异丙肾上腺素(ISO)诱导的高血脂小鼠(C57BL/6J)中,观察到肠道通透性受损,粪便中 C.sp 的减少和 SFA 的增加得到进一步验证。最后,在 ISO 诱导的高血脂小鼠体内补充 C.sp 后,心脏功能、纤维化和心肌大小得到了部分缓解,循环中的 SFA 水平也有所下降。结论梭状芽孢杆菌在高房颤动中的丰度增加,通过下调循环 SFA 水平来补偿心脏功能的恶化。研究结果表明,肠道微生物群-SFA 轴在心房颤动保护中起着重要作用,这可能为开发心房颤动的益生菌疗法提供战略优势。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


