Understanding the Vibrational Structure and Ultrafast Dynamics of the Metal Carbonyl Precatalyst [Mn(ppy)(CO)4]
IF 3.7
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
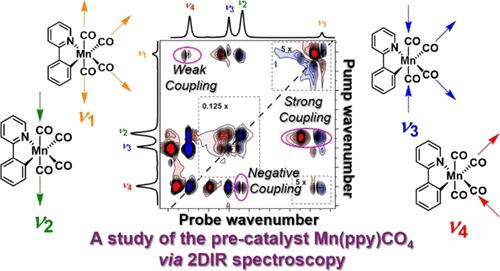
The solution phase structure, vibrational spectroscopy, and ultrafast relaxation dynamics of the precatalyst species [Mn(ppy)(CO)4] (1) in solution have been investigated using ultrafast two-dimensional infrared (2D-IR) spectroscopy. By comparing 2D-IR data with the results of anharmonic density functional theory (DFT) calculations, we establish an excellent agreement between measured and predicted inter-mode couplings of the carbonyl stretching vibrational modes of 1 that relates to the atomic displacements of axial and equatorial ligands in the modes and the nature of the molecular orbitals involved in M–CO bonding. Measurements of IR pump–probe spectra and 2D-IR spectra as a function of waiting time reveal the presence of ultrafast (few ps) intramolecular vibrational energy redistribution between carbonyl stretching modes prior to vibrational relaxation. The vibrational relaxation times of the CO-stretching modes of 1 are found to be relatively solvent-insensitive, suggestive of limited solvent–solute interactions in the ground electronic state. Overall, these data provide a detailed picture of the complex potential energy surface, bonding and vibrational dynamics of 1, establishing a fundamental basis for the next steps in understanding and modulating precatalyst behavior.
了解金属羰基前催化剂 [Mn(ppy)(CO)4] 的振动结构和超快动力学特性
我们利用超快二维红外光谱(2D-IR)研究了前催化剂物种 [Mn(ppy)(CO)4] (1) 在溶液中的溶液相结构、振动光谱和超快弛豫动力学。通过比较二维红外光谱数据和非谐波密度泛函理论(DFT)计算结果,我们发现 1 的羰基伸缩振动模式的测量值和预测值的模式间耦合非常吻合,这与模式中轴向和赤道配体的原子位移以及 M-CO 键合所涉及的分子轨道的性质有关。红外泵探头光谱和二维红外光谱随等待时间变化的测量结果表明,在振动弛豫之前,羰基伸展模式之间存在超快(几 ps)的分子内振动能量再分配。研究发现,1 的 CO 拉伸模式的振动弛豫时间对溶剂相对不敏感,这表明在基态电子状态下溶剂与溶质之间的相互作用是有限的。总之,这些数据详细描绘了 1 的复杂势能面、成键和振动动力学,为下一步了解和调节前催化剂行为奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.70
自引率
0.00%
发文量
0
期刊介绍:
ACS Physical Chemistry Au is an open access journal which publishes original fundamental and applied research on all aspects of physical chemistry. The journal publishes new and original experimental computational and theoretical research of interest to physical chemists biophysical chemists chemical physicists physicists material scientists and engineers. An essential criterion for acceptance is that the manuscript provides new physical insight or develops new tools and methods of general interest. Some major topical areas include:Molecules Clusters and Aerosols; Biophysics Biomaterials Liquids and Soft Matter; Energy Materials and Catalysis

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


