Cas1 mediates the interference stage in a phage-encoded CRISPR–Cas system
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Clustered regularly interspaced short palindromic repeats (CRISPR)–Cas systems are prokaryotic adaptive immune systems against invading phages and other mobile genetic elements. Notably, some phages, including the Vibrio cholerae-infecting ICP1 (International Center for Diarrheal Disease Research, Bangladesh cholera phage 1), harbor CRISPR–Cas systems to counteract host defenses. Nevertheless, ICP1 Cas8f lacks the helical bundle domain essential for recruitment of helicase-nuclease Cas2/3 during target DNA cleavage and how this system accomplishes the interference stage remains unknown. Here, we found that Cas1, a highly conserved component known to exclusively work in the adaptation stage, also mediates the interference stage through connecting Cas2/3 to the DNA-bound CRISPR-associated complex for antiviral defense (Cascade; CRISPR system yersinia, Csy) of the ICP1 CRISPR–Cas system. A series of structures of Csy, Csy–dsDNA (double-stranded DNA), Cas1–Cas2/3 and Csy–dsDNA–Cas1–Cas2/3 complexes reveal the whole process of Cas1-mediated target DNA cleavage by the ICP1 CRISPR–Cas system. Together, these data support an unprecedented model in which Cas1 mediates the interference stage in a phage-encoded CRISPR–Cas system and the study also sheds light on a unique model of primed adaptation. The ICP1 (International Center for Diarrheal Disease Research, Bangladesh cholera phage 1) clustered regularly interspaced short palindromic repeats (CRISPR)–Cas system, which lacks the helical bundle domain in Cas8f, uses Cas1 to mediate the interference stage by connecting Cas2/3 to the DNA-bound CRISPR-associated complex for antiviral defense (Cascade).
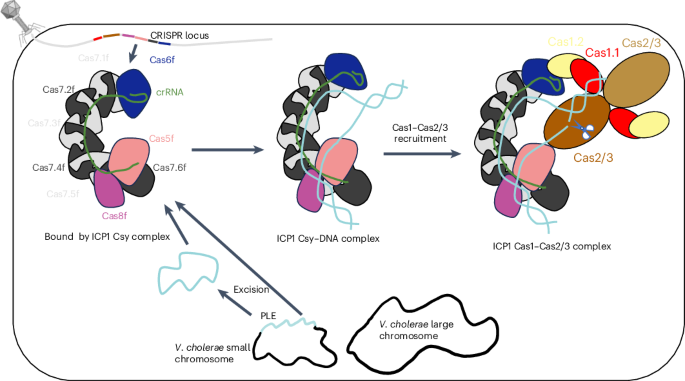
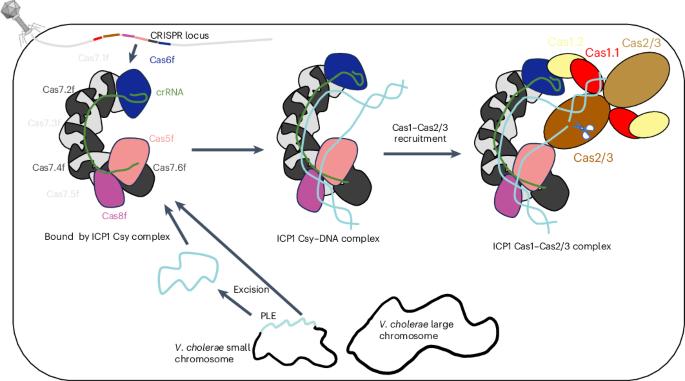
Cas1 在噬菌体编码的 CRISPR-Cas 系统中介导干扰阶段
簇状有规律间隔短回文重复序列(CRISPR)-Cas系统是原核生物对抗入侵噬菌体和其他移动遗传因子的适应性免疫系统。值得注意的是,一些噬菌体,包括感染霍乱弧菌的ICP1(国际腹泻病研究中心,孟加拉霍乱噬菌体1),都携带CRISPR-Cas系统,以对抗宿主的防御。然而,ICP1的Cas8f缺乏在靶DNA裂解过程中招募螺旋酶-核酸酶Cas2/3所必需的螺旋束结构域,该系统如何完成干扰阶段仍是未知数。在这里,我们发现,已知只在适应阶段发挥作用的高度保守成分Cas1也通过连接Cas2/3和ICP1 CRISPR-Cas系统中与DNA结合的抗病毒防御CRISPR相关复合物(Cascade; CRISPR system yersinia, Csy)来介导干扰阶段。Csy、Csy-dsDNA(双链DNA)、Cas1-Cas2/3和Csy-dsDNA-Cas1-Cas2/3复合物的一系列结构揭示了ICP1 CRISPR-Cas系统介导的Cas1裂解靶DNA的全过程。这些数据共同支持了一个前所未有的模型,在该模型中,Cas1介导了噬菌体编码的CRISPR-Cas系统中的干扰阶段,该研究还揭示了一个独特的引物适应模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


