The solvation environment of molecularly dispersed cobalt phthalocyanine determines methanol selectivity during electrocatalytic CO2 reduction
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Heterogenized molecular electrocatalysts are a promising group of materials that can electrocatalytically convert waste molecules into higher-value products. However, how the dispersion state of molecules affects the catalytic process is not well understood. Using cobalt phthalocyanine (CoPc) dispersed on carbon nanotubes (CNTs) as a model system, here we show that increasing the direct interaction of the molecular catalyst with cations notably enhances the CO2 reduction reaction. Specifically, molecularly dispersed CoPc on CNTs yields an eightfold increase in methanol selectivity compared with aggregated CoPc on CNTs. In situ spectroscopic studies confirm the presence of two intermediates located at different positions of the double layer. Density functional theory calculations further reveal that CoPc molecules inside the Stern layer are active for methanol production due to the direct interaction with cations. Similar enhancement effects are also observed for other reactions, showing that dispersing molecular catalysts into monomeric states is a general design parameter. Heterogenized molecular catalysts provide a means to add well-defined active sites to electrode surfaces. Here, using cobalt phthalocyanine on carbon nanotubes in cation-mediated CO2 reduction, the mechanism of molecular dispersion and its impact on rate and product selectivity are revealed.
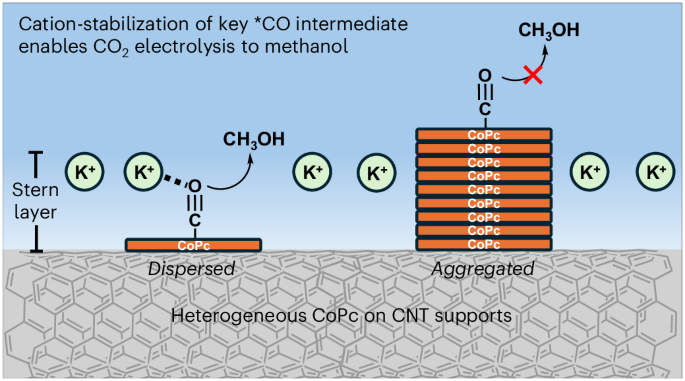
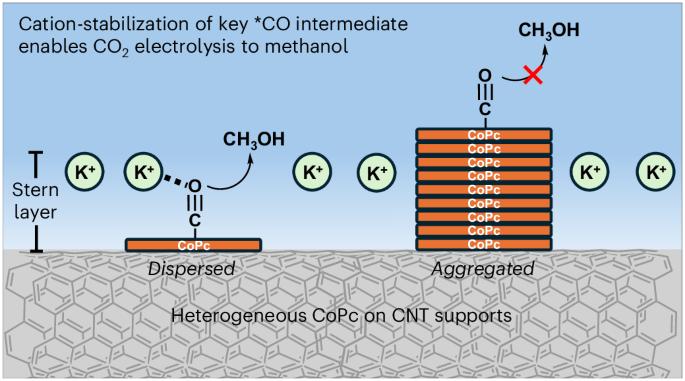
分子分散酞菁钴的溶解环境决定了电催化二氧化碳还原过程中的甲醇选择性
异质化分子电催化剂是一类前景广阔的材料,可通过电催化将废弃分子转化为高价值产品。然而,人们对分子的分散状态如何影响催化过程还不甚了解。以分散在碳纳米管(CNT)上的酞菁钴(CoPc)为模型系统,我们在此表明,增加分子催化剂与阳离子的直接相互作用可显著增强二氧化碳还原反应。具体来说,与聚合在碳纳米管上的 CoPc 相比,分子分散在碳纳米管上的 CoPc 对甲醇的选择性提高了八倍。原位光谱研究证实,在双层的不同位置存在两种中间产物。密度泛函理论计算进一步表明,由于与阳离子的直接相互作用,斯特恩层内的 CoPc 分子对甲醇生产具有活性。在其他反应中也观察到了类似的增强效应,这表明将分子催化剂分散到单体状态是一种通用的设计参数。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


