Multifunctional Nanodrug-Mediated Immunotherapy in Microsatellite Stable Colorectal Cancer via Promoting m6A Modification and M1-Like Tumor-Associated Macrophages Polarization
引用次数: 0
Abstract
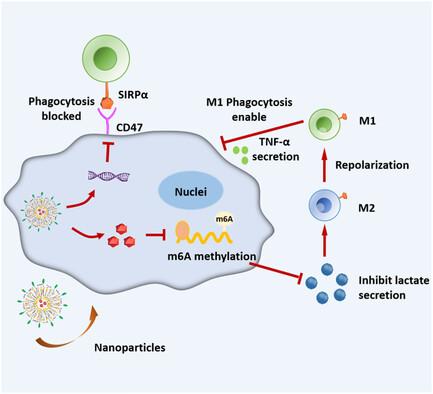
Immunotherapy has made great progress in various solid tumors. However, the “cold” tumor immune microenvironment of microsatellite stable subtype colorectal cancer (MSS-CRC) hinders the effectiveness of immunotherapy. Therefore, reshaping the immunosuppressive microenvironment and initiating efficient antitumor immune responses are critical for immunotherapy of MSS-CRC. According to the analysis of clinical samples, it is found that the levels of fat mass and obesity-associated protein (FTO) and M2-like tumor-associated macrophages (TAMs) infiltration are significantly elevated in CRC tissue, which has driven one to construct a targeted cationic liposome to simultaneously enhance the RNA methylation and inhibit the CD47 immune checkpoint expression of tumor cells in the hope of promoting the M1-like TAMs polarization and phagocytosis. By upregulating the m6A modification of tumor cells, the lactate secretion is decreased to promote the TAMs repolarized into M1-like. Meanwhile, CD47 siRNA codelivered by the cationic liposomes downregulates the expression of immune checkpoint CD47 on the cancer cell surface, which enhances the phagocytic ability of the M1-like TAMs. The combination treatment scheme is expected to provide a new option for treating MSS-CRC, which may also be extended for treating other immunologically “cold” tumors.
多功能纳米药物通过促进 m6A 修饰和 M1 型肿瘤相关巨噬细胞极化对微卫星稳定型结直肠癌进行免疫治疗
免疫疗法在各种实体瘤中取得了重大进展。然而,微卫星稳定亚型结直肠癌(MSS-CRC)"冰冷 "的肿瘤免疫微环境阻碍了免疫疗法的有效性。因此,重塑免疫抑制微环境并启动高效的抗肿瘤免疫反应对 MSS-CRC 的免疫治疗至关重要。根据对临床样本的分析发现,CRC组织中的脂肪量和肥胖相关蛋白(FTO)以及M2样肿瘤相关巨噬细胞(TAMs)浸润水平显著升高,这促使人们构建一种靶向阳离子脂质体,同时增强肿瘤细胞的RNA甲基化和抑制CD47免疫检查点的表达,希望能促进M1样TAMs的极化和吞噬。通过上调肿瘤细胞的 m6A 修饰,减少乳酸分泌,促进 TAMs 重极化为 M1 样。同时,阳离子脂质体编码递送的 CD47 siRNA 可下调癌细胞表面免疫检查点 CD47 的表达,从而增强 M1 样 TAMs 的吞噬能力。这种联合治疗方案有望为治疗MSS-CRC提供一种新的选择,也可用于治疗其他免疫学上的 "冷门 "肿瘤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


