Structure-Guided Antiviral Peptides Identification Targeting the HIV-1 Integrase
IF 3.7
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
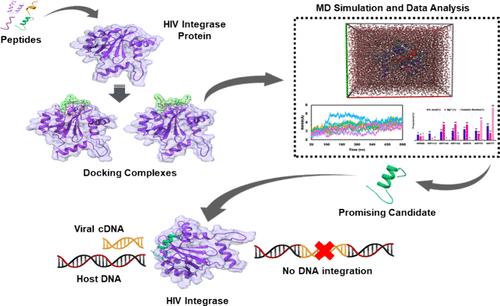
HIV-1 integrase (IN), a major protein in the HIV life cycle responsible for integrating viral cDNA into the host DNA, represents a promising drug target. Small peptides have emerged as antiviral therapeutics for HIV because of their facile synthesis, highly selective nature, and fewer side effects. However, selecting the best candidates from a vast pool of peptides is a daunting task. In this study, multistep virtual screening was employed to identify potential peptides from a list of 280 HIV inhibitory peptides. Initially, 80 peptides were selected based on their minimum inhibitory concentrations (MIC). Then, molecular docking was performed to evaluate their binding scores compared to HIP000 and HIP00N which are experimentally validated HIV-1 integrase binding peptides that were used as a positive and negative control, respectively. The top-scoring docked complexes, namely, IN-HIP1113, IN-HIP1140, IN-HIP1142, IN-HIP678, IN-HIP776, and IN-HIP777, were subjected to initial 500 ns molecular dynamics (MD) simulations. Subsequently, HIP776, HIP777, and HIP1142 were selected for an in-depth mechanistic study of peptide interactions, with multiple simulations conducted for each complex spanning one microsecond. Independent simulations of the peptides, along with comparisons to the bound state, were performed to elucidate the conformational dynamics of the peptides. These peptides exhibit strong interactions with specific residues, as revealed by snapshot interaction analysis. Notably, LYS159, LYS156, VAL150, and GLU69 residues are prominently involved in these interactions. Additionally, residue-based binding free energy (BFE) calculations highlight the significance of HIS67, GLN148, GLN146, and SER147 residues within the binding pocket. Furthermore, the structure–activity relationship (SAR) analysis demonstrated that aromatic amino acids and the overall volume of peptides are the two major contributors to the docking scores. The best peptides will be validated experimentally by incorporating SAR properties, aiming to develop them as therapeutic agents and structural models for future peptide-based HIV-1 drug design, addressing the urgent need for effective HIV treatments.
以 HIV-1 整合酶为目标的结构引导型抗病毒肽鉴定
HIV-1 整合酶(IN)是 HIV 生命周期中的一种主要蛋白质,负责将病毒 cDNA 整合到宿主 DNA 中。小肽因其合成简便、选择性强、副作用小等特点,已成为艾滋病病毒的抗病毒疗法。然而,从大量多肽中选择最佳候选药物是一项艰巨的任务。本研究采用多步虚拟筛选法,从 280 种抑制艾滋病病毒的多肽中找出潜在的多肽。首先,根据肽的最低抑制浓度(MIC)筛选出 80 种肽。HIP000 和 HIP00N 是实验验证的 HIV-1 整合酶结合肽,分别用作阳性对照和阴性对照。对得分最高的对接复合物,即 IN-HIP1113、IN-HIP1140、IN-HIP1142、IN-HIP678、IN-HIP776 和 IN-HIP777 进行了最初的 500 ns 分子动力学(MD)模拟。随后,选择 HIP776、HIP777 和 HIP1142 对肽的相互作用进行了深入的机理研究,对每个复合物进行了多次模拟,模拟时间跨度为一微秒。对多肽进行了独立模拟,并与结合态进行了比较,以阐明多肽的构象动态。快照相互作用分析表明,这些多肽与特定残基有很强的相互作用。值得注意的是,LYS159、LYS156、VAL150 和 GLU69 残基明显参与了这些相互作用。此外,基于残基的结合自由能(BFE)计算突显了 HIS67、GLN148、GLN146 和 SER147 残基在结合口袋中的重要性。此外,结构-活性关系(SAR)分析表明,芳香族氨基酸和肽的总体积是影响对接得分的两个主要因素。我们将结合 SAR 特性对最佳多肽进行实验验证,旨在将它们开发成治疗剂和结构模型,用于未来基于多肽的 HIV-1 药物设计,满足对有效治疗 HIV 的迫切需求。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.70
自引率
0.00%
发文量
0
期刊介绍:
ACS Physical Chemistry Au is an open access journal which publishes original fundamental and applied research on all aspects of physical chemistry. The journal publishes new and original experimental computational and theoretical research of interest to physical chemists biophysical chemists chemical physicists physicists material scientists and engineers. An essential criterion for acceptance is that the manuscript provides new physical insight or develops new tools and methods of general interest. Some major topical areas include:Molecules Clusters and Aerosols; Biophysics Biomaterials Liquids and Soft Matter; Energy Materials and Catalysis

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


