A Highly Charged Positive Cage Causes Simultaneous Enhancement of Type-II and O2-Independent-Type-I Photodynamic Therapy via One-/Two-Photon Stimulation and Tumor Immunotherapy via PANoptosis and Ferroptosis
IF 11.1
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
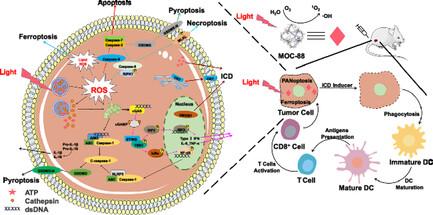
To solve the oxygen dependence problem of photodynamic therapy (PDT), it is critical to explore photosensitizers that do not rely on O2 molecule to generate reactive oxygen species (ROS). Herein, a stable cationic metal-organic cage [Pd6(RuLoz3)8](BF4)28 (MOC-88) that possesses high +28 charges is designed. The cage-confined positive microenvironment enables efficient generation of hydroxyl radicals and improved yield of the singlet oxygen under one-/two-photon excitation, showing excellent performance to concurrently enhance Type-II and O2-independent-Type-I PDT. Moreover, the effective ROS production and robust lipid peroxidation trigger a series of signaling pathways (inflammasome, cyclic guanosine monophosphate–adenosine monophosphate synthase stimulator of interferon genes, and NF-κB) to evoke PANoptosis and ferroptosis in tumor cells, enabling MOC-88 to simultaneously cause the loss of cell membrane integrity, release a series of inflammatory cytokines and damage-associated molecular patterns, stimulate the maturation and antigen presentation ability of dendritic cells, and ultimately activate T-cell-dependent adaptive immunity in vivo to inhibit tumor growth.
高电荷正笼子通过单/双光子刺激和泛凋亡与铁凋亡同时增强 II 型和 O2 依赖性 I 型光动力疗法和肿瘤免疫疗法
为解决光动力疗法(PDT)的氧依赖性问题,探索不依赖氧气分子产生活性氧(ROS)的光敏剂至关重要。本文设计了一种具有高 +28 电荷的稳定阳离子金属有机笼 [Pd6(RuLoz3)8](BF4)28(MOC-88)。笼状封闭的正性微环境能在单/双光子激发下高效产生羟自由基并提高单线态氧的产量,在同时增强 II 型和不依赖于 O2 的 I 型 PDT 方面表现出卓越的性能。此外,有效的 ROS 生成和强大的脂质过氧化还能触发一系列信号通路(炎性体、环磷酸鸟苷-单磷酸腺苷合成酶、干扰素基因刺激器和 NF-κB),从而唤起肿瘤细胞的泛凋亡和铁凋亡、MOC-88 可同时导致细胞膜完整性丧失,释放一系列炎性细胞因子和损伤相关分子模式,刺激树突状细胞的成熟和抗原递呈能力,最终激活体内依赖 T 细胞的适应性免疫,从而抑制肿瘤生长。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


