Acid-base synergistic effect towards catalytic transfer hydrogenation reactions
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Catalytic transfer hydrogenation (CTH) of aldehydes/ketones by using alcohols as hydrogen donors is an attractive and environmentally friendly hydrogenation technology, which has evoked widespread attention in synthesis of fine chemicals from biomass resources. In this work, a Co-Al mixed metal oxide catalyst (CoAl-MMO-200) was obtained through structural topological transformation process of layered double hydroxides, which was used in the CTH reaction of furfural with isopropanol. Notably, the reaction rate achieves 0.023 mol g h, which is preponderate to acid-base catalysts earlier reported. The joint research based on CO-TPD, NH-TPD and poisoning experiment substantiates that the synergy effect of acid-base sites plays an essential role in this catalytic system. Isotopic labelling MS, FT-IR and DFT studies further verify that the CTH reaction of furfural occurs the Meerwein-Ponndorf-Verley (MPV) route, in which the basic site promotes the deprotonation of isopropanol whilst the acid site facilitates the formation of a six-membered ring transition state. This work demonstrates an acid-base synergetic catalysis for the CTH reaction by revealing the structure-property correlation and exploring reaction route, which is instructive for designing high-performance heterogenous catalysts.
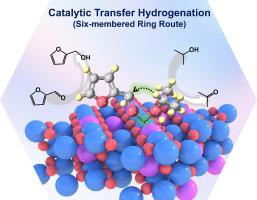
催化转移加氢反应的酸碱协同效应
以醇为供氢体进行醛/酮催化转移加氢(CTH)是一种极具吸引力的环保型加氢技术,在利用生物质资源合成精细化学品方面引起了广泛关注。本研究通过层状双氢氧化物的结构拓扑变换过程获得了 Co-Al 混合金属氧化物催化剂(CoAl-MMO-200),并将其用于糠醛与异丙醇的 CTH 反应。值得注意的是,该催化剂的反应速率达到了 0.023 mol g h,优于之前报道的酸碱催化剂。基于 CO-TPD、NH-TPD 和中毒实验的联合研究证实,酸碱位点的协同效应在该催化体系中起着至关重要的作用。同位素标记 MS、傅立叶变换红外光谱和 DFT 研究进一步验证了糠醛的 CTH 反应是通过 Meerwein-Ponndorf-Verley (MPV) 途径进行的,其中碱性位点促进了异丙醇的去质子化,而酸性位点则促进了六元环过渡态的形成。这项工作通过揭示结构-性能相关性和探索反应路线,证明了 CTH 反应的酸碱协同催化,对设计高性能异源催化剂具有指导意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


