Substrate specificity profiling of heat-sensitive serine protease from the fungus Onygena corvina
IF 3.3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Proteases catalyze hydrolysis of amide bonds within peptides and proteins, therefore they play crucial functions for organism functioning, but also in industry to facilitate numerous processes. Feather-degrading fungus Onygena corvina (O. corvina) is loaded with numerous proteases that can be utilized for variety of applications. The most active species of these enzymes is heat-sensitive serine protease (NHSSP), from O. corvina fungi and due to its potential applications in industry is an alternative to proteinase K. The uniqueness of NHSSP relies on the ability of this enzyme to hydrolyze peptides at neutral to acidic pH values between 5.0 and 8.5, with an optimum of 6.8 and a temperature activity ranging from 15 to 50 °C making NHSSP exceptionally universal enzyme.
Thus, we have performed the in-depth characterization of NHSSP substrate specificity by using a positional scanning substrate combinatorial library (PS-SCL). Afterward, we obtained a set of fluorescent substrates hydrolyzed by NHSSP that served as a leading sequence for the first tailored covalent inhibitor of this enzyme, containing a diphenylphosphonate as a warhead and MeOSuc amine protecting group. Our first inhibitor for NHSSP binds potently with target protease and is a tool for future study of this enzyme functions.
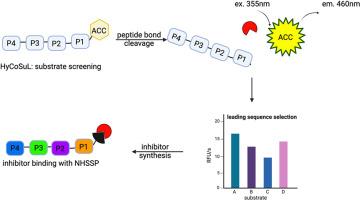
姬松茸真菌热敏丝氨酸蛋白酶的底物特异性分析。
蛋白酶能催化肽和蛋白质中酰胺键的水解,因此对生物体的功能起着至关重要的作用,在工业中也能促进许多工艺流程。羽毛降解真菌 Onygena corvina(O. corvina)含有多种蛋白酶,可用于多种用途。NHSSP 的独特之处在于它能在 5.0 到 8.5 之间的中性到酸性 pH 值范围内水解肽,最适 pH 值为 6.8,温度活性范围为 15-50°C,这使得 NHSSP 成为一种特别通用的酶。因此,我们利用位置扫描底物组合库(PS-SCL)对 NHSSP 的底物特异性进行了深入表征。随后,我们获得了一组 NHSSP 可水解的荧光底物,这些荧光底物可作为首个定制的共价抑制剂的前导序列,该抑制剂含有二苯基膦酸盐作为弹头和 MeOSuc 氨基保护基团。我们的首个 NHSSP 抑制剂能与目标蛋白酶有效结合,是未来研究该酶功能的工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimie
生物-生化与分子生物学
CiteScore
7.20
自引率
2.60%
发文量
219
审稿时长
40 days
期刊介绍:
Biochimie publishes original research articles, short communications, review articles, graphical reviews, mini-reviews, and hypotheses in the broad areas of biology, including biochemistry, enzymology, molecular and cell biology, metabolic regulation, genetics, immunology, microbiology, structural biology, genomics, proteomics, and molecular mechanisms of disease. Biochimie publishes exclusively in English.
Articles are subject to peer review, and must satisfy the requirements of originality, high scientific integrity and general interest to a broad range of readers. Submissions that are judged to be of sound scientific and technical quality but do not fully satisfy the requirements for publication in Biochimie may benefit from a transfer service to a more suitable journal within the same subject area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


