Modulation of the Meisenheimer complex metabolism of nitro-benzothiazinones by targeted C-6 substitution
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Tuberculosis, caused by Mycobacterium tuberculosis, remains a major public health concern, demanding new antibiotics with innovative therapeutic principles due to the emergence of resistant strains. Benzothiazinones (BTZs) have been developed to address this problem. However, an unprecedented in vivo biotransformation of BTZs to hydride-Meisenheimer complexes has recently been discovered. Herein, we present a study of the influence of electron-withdrawing groups on the propensity of HMC formation in whole cells for a series of C-6-substituted BTZs obtained through reductive fluorocarbonylation as a late-stage functionalization key step. Gibbs free energy of reaction and Mulliken charges and Fukui indices on C-5 at quantum mechanics level were found as good indicators of in vitro HMC formation propensity. These results provide a first blueprint for the evaluation of HMC formation in drug development and set the stage for rational pharmacokinetic optimization of BTZs and similar drug candidates. Benzothiazinones (BTZs) are being developed as new antibiotics against the infection caused by Mycobacterium tuberculosis, however, BTZs can undergo an in vivo biotransformation to hydride-Meisenheimer complexes (HMC). Here, the authors show that HMC formation can be modulated by C-6 substitution of BTZ.
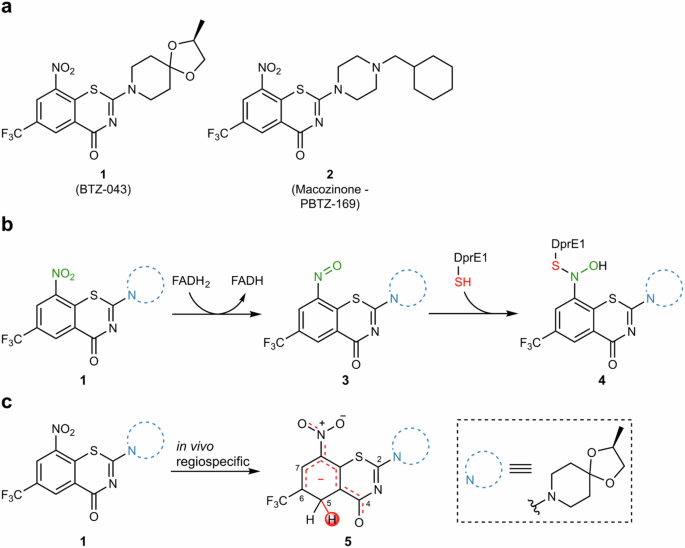
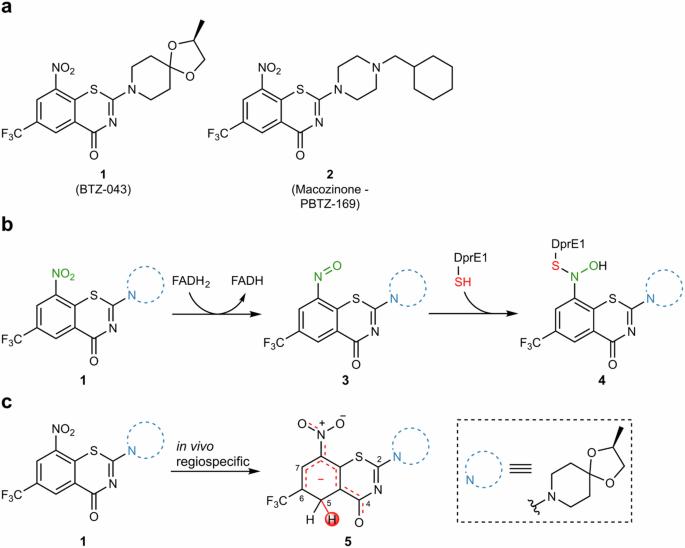
通过有针对性的 C-6 取代调节硝基苯并噻嗪酮的迈森海默复合代谢。
由结核分枝杆菌引起的结核病仍然是一个重大的公共卫生问题,由于耐药菌株的出现,需要具有创新治疗原理的新型抗生素。苯并噻嗪酮(BTZs)就是为解决这一问题而开发的。然而,最近发现了一种前所未有的 BTZs 向氢化物-梅森海默复合物的体内生物转化。在此,我们研究了作为后期官能化关键步骤,通过还原氟羰基化获得的一系列 C-6 取代 BTZ 在全细胞中形成氢化物-梅森海默复合物的倾向受电子抽离基团的影响。量子力学水平上的反应吉布斯自由能以及 C-5 上的 Mulliken 电荷和 Fukui 指数是体外 HMC 形成倾向的良好指标。这些结果为在药物开发过程中评估 HMC 的形成提供了第一个蓝图,并为 BTZs 和类似候选药物的合理药代动力学优化奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


