PDK4-mediated Nrf2 inactivation contributes to oxidative stress and diabetic kidney injury
Abstract
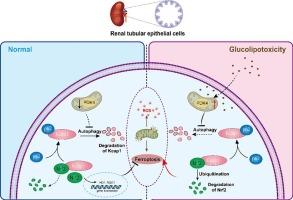
Diabetic kidney disease (DKD) is often featured with redox dyshomeostatis. Pyruvate dehydrogenase kinase 4 (PDK4) is the hub for DKD development. However, the mechanism by which PDK4 mediates DKD is poorly understood. The current work aimed to elucidate the relationship between PDK4 and DKD from the perspective of redox manipulation. Oxidative stress was observed in the human proximal tubular cell line (HK-2 cells) treated with a high concentration of glucose and palmitic acid (HGL). The mechanistic study showed that PDK4 could upregulate Kelch-like ECH-associated protein 1 (Keap1) in HGL-treated HK-2 cells through the suppression of autophagy, resulting in the depletion of nuclear factor erythroid 2-related factor 2 (Nrf2), the master regulator of redox homeostasis. At the cellular level, pharmacological inhibition or genetic knockdown of PDK4 could boost Nrf2, followed by the increase of a plethora of antioxidant enzymes and ferroptosis-suppression enzymes. Meanwhile, the inhibition or knockdown of PDK4 remodeled iron metabolism, further mitigating oxidative stress and lipid peroxidation. The same trend was observed in the DKD mice model. The current work highlighted the role of PDK4 in the development of DKD and suggested that PDK4 might be a promising target for the management of DKD.

| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


