Early symptom-associated inflammatory responses shift to type 2 responses in controlled human schistosome infection
IF 17.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Schistosomiasis is an infection caused by contact with Schistosoma-contaminated water and affects more than 230 million people worldwide with varying morbidity. The roles of T helper 2 (TH2) cells and regulatory immune responses in chronic infection are well documented, but less is known about human immune responses during acute infection. Here, we comprehensively map immune responses during controlled human Schistosoma mansoni infection using male or female cercariae. Immune responses to male or female parasite single-sex infection were comparable. An early TH1-biased inflammatory response was observed at week 4 after infection, which was particularly apparent in individuals experiencing symptoms of acute schistosomiasis. By week 8 after infection, inflammatory responses were followed by an expansion of TH2 and regulatory cell subsets. This study demonstrates the shift from TH1 to both TH2 and regulatory responses, typical of chronic schistosomiasis, in the absence of egg production and provides immunological insight into the clinical manifestations of acute schistosomiasis.
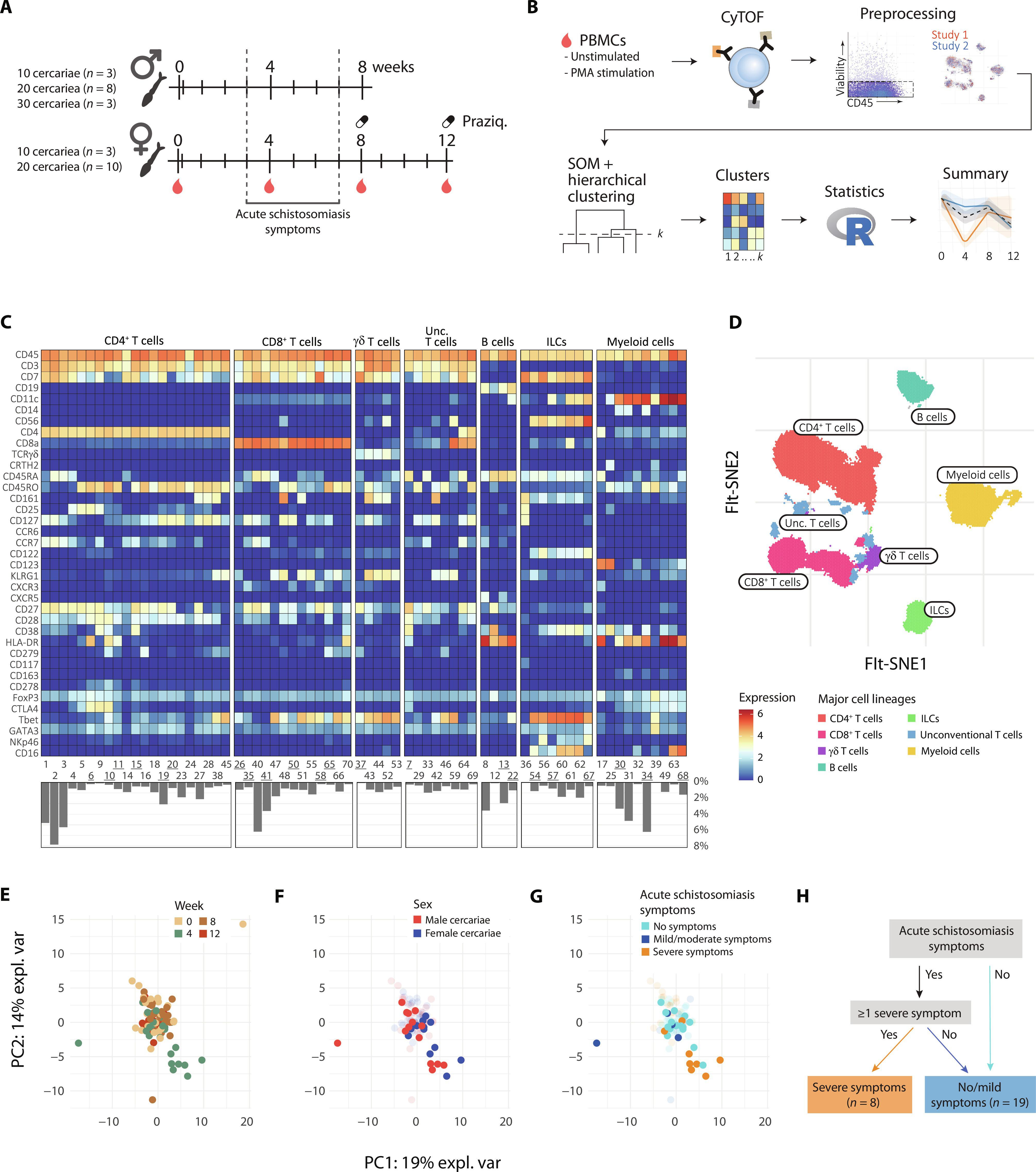
在控制人类血吸虫感染的过程中,早期症状相关炎症反应转变为 2 型反应。
血吸虫病是一种因接触被血吸虫污染的水而引起的感染,全球有超过 2.3 亿人受到影响,发病率各不相同。T 辅助细胞 2(TH2)和调节性免疫反应在慢性感染中的作用有据可查,但对急性感染期间的人体免疫反应却知之甚少。在这里,我们利用雄性或雌性carcariae全面描绘了人类曼氏血吸虫受控感染期间的免疫反应。雄性或雌性寄生虫单性感染时的免疫反应相当。在感染后第 4 周观察到早期 TH1 偏重的炎症反应,这在出现急性血吸虫病症状的个体中尤为明显。到感染后第 8 周,炎症反应后 TH2 和调节细胞亚群扩大。这项研究表明,在没有产卵的情况下,慢性血吸虫病的典型反应是从TH1转变为TH2和调节反应,并为急性血吸虫病的临床表现提供了免疫学见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Immunology
Immunology and Microbiology-Immunology
CiteScore
32.90
自引率
2.00%
发文量
183
期刊介绍:
Science Immunology is a peer-reviewed journal that publishes original research articles in the field of immunology. The journal encourages the submission of research findings from all areas of immunology, including studies on innate and adaptive immunity, immune cell development and differentiation, immunogenomics, systems immunology, structural immunology, antigen presentation, immunometabolism, and mucosal immunology. Additionally, the journal covers research on immune contributions to health and disease, such as host defense, inflammation, cancer immunology, autoimmunity, allergy, transplantation, and immunodeficiency. Science Immunology maintains the same high-quality standard as other journals in the Science family and aims to facilitate understanding of the immune system by showcasing innovative advances in immunology research from all organisms and model systems, including humans.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


